Synthesis of Sterically Congested Carbonyl Compounds via an ipso-Selective Sulfonium Rearrangement
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Despite the success of metal-catalyzed cross-coupling strategies to assemble a variety of C–C bonds, the synthesis of sterically congested α-arylated carbonyl compounds remains a difficult task. Herein, we present an ipso-rearrangement approach relying on electron redistribution in sulfonium intermediates. This modular method enables the synthesis of a variety of α-arylated carbonyl compounds and is orthogonal to traditional metal-catalyzed cross-coupling strategies. More importantly, it demonstrated remarkable robustness toward steric hindrance and allowed us to efficiently forge congested C–C bonds.
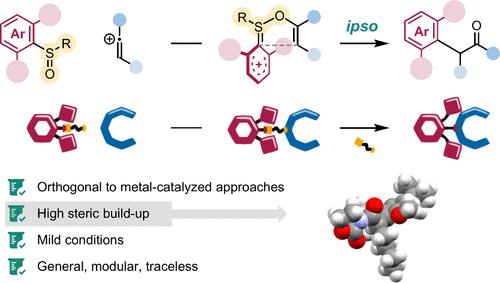
通过超选择性磺胺重排合成空间密集羰基化合物
尽管金属催化的交叉偶联策略成功地组装了各种C-C键,但合成空间拥挤的α-芳基化羰基化合物仍然是一项艰巨的任务。在此,我们提出了一种依赖于磺胺中间体中电子重分布的ipso重排方法。这种模块化方法可以合成多种α-芳基化羰基化合物,并且与传统的金属催化交叉偶联策略正交。更重要的是,它对位阻表现出了显著的鲁棒性,使我们能够有效地形成拥挤的C-C键。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


