Hecogenin‑nitrogen mustard hybrids with improved anti-breast cancer activity: Design, synthesis, and biological evaluation
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
To enhance the efficacy of hecogenin (HCG) against breast cancer cells, we designed and synthesized two series of new HCG‑nitrogen mustard hybrids (4a–4f and 5a–5f) by linking benzoic acid mustard or chlorambucil to HCG via amino acid residues. The derivatives were screened to assess their anti-proliferative activity against three human breast cancer cell lines (MDA-MB-231, MDA-MB-468, and MCF-7), and one normal human breast MCF-10A cell line. Among the synthesized compounds, hybrid 5d exhibited the most potent anti-proliferative activity against the triple-negative breast cancer cell line MDA-MB-231, with an IC50 value of 2.2 μM. This represents a 27.2-fold increase in potency compared to the parent compound HCG (IC50 = 59.8 μM). Furthermore, hybrid 5d exhibited low toxicity toward MCF-10A cells (IC50 > 100 μM), indicating certain selectivity. Notably, the transwell migration assay revealed that hybrid 5d significantly inhibited the invasion of MDA-MB-231 cells. Preliminary mechanism studies indicated that hybrid 5d induced G2/M phase arrest and apoptosis via the mitochondria-related apoptotic pathway, as well as caused DNA damage. Collectively, these results suggest that hybrid 5d is a promising lead compound for anti-breast cancer research worthy of further investigation.
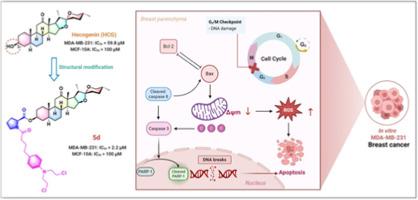
具有改善抗乳腺癌活性的异源原-氮芥杂交:设计、合成和生物学评价。
为了提高HCG (heocgenin, HCG)对乳腺癌细胞的抗肿瘤作用,我们设计并合成了两个新的HCG -氮芥菜杂种(4a-4f和5a-5f),通过氨基酸残基将苯甲酸芥菜或氯霉素与HCG连接。对三种人乳腺癌细胞系(MDA-MB-231、MDA-MB-468和MCF-7)和一种正常人乳腺癌细胞系MCF-10A进行了抑增殖活性筛选。在合成的化合物中,杂合物5d对三阴性乳腺癌细胞株MDA-MB-231的抗增殖活性最强,IC50值为2.2 μM。与母体化合物HCG (IC50 = 59.8 μM)相比,效价提高了27.2倍。此外,杂交5d对MCF-10A细胞具有低毒性(IC50 ~ 100 μM),表明具有一定的选择性。值得注意的是,transwell迁移实验显示,杂交5d显著抑制MDA-MB-231细胞的侵袭。初步机制研究表明,杂交5d通过线粒体相关凋亡途径诱导G2/M期阻滞和细胞凋亡,并引起DNA损伤。综上所述,这些结果表明混合5d是一种有前景的抗乳腺癌先导化合物,值得进一步研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


