Synthesis and Docking Study of 6-Substituted Indolo[2,3-b]quinoxalines
IF 0.9
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
6-(2-Bromoethyl)-6H-indolo[2,3-b]quinoxaline was synthesized and reacted with arylamines to obtain N-(2-[6H-indolo[2,3-b]quinoxalin-6-yl)ethyl]arylamines and N,N-disubstituted 4-methylaniline. Previosly unknown 6-vinyl- and 6-(2-azidoethyl) derivatives of 6-(2-bromoethyl)-6H-indolo[2,3-b]quinoxaline and indolo[2,3-b]quinoxaline–pyrimidine and 1,2-bis(6H-indolo[2,3-b]quinoxalin-6-yl)ethane hybrids were also synthesized. Molecular docking studies of the synthesized indolo[2,3-b]quinoxaline derivatives against 4 different receptors indicated high predicted activity for the indoloquinoxaline–pyrimidine conjugate.
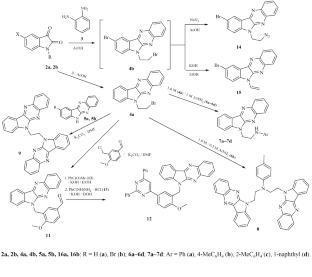
6-取代吲哚[2,3-b]喹啉类化合物的合成及对接研究
合成了6-(2-溴乙基)- 6h -吲哚[2,3-b]喹啉,并与芳胺反应得到N-(2-[6h -吲哚[2,3-b]喹啉-6-基)乙基]芳胺和N,N-二取代4-甲基苯胺。还合成了以前未知的6-(2-溴乙基)- 6h -吲哚[2,3-b]喹诺啉的6-乙烯基和6-(2-叠氮乙基)衍生物和吲哚[2,3-b]喹诺啉嘧啶和1,2-二(6h -吲哚[2,3-b]喹诺啉-6-基)乙烷杂化物。对合成的吲哚[2,3-b]喹诺啉衍生物与4种不同受体的分子对接研究表明,吲哚喹诺啉-嘧啶缀合物具有较高的预测活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


