Synthesis of Functionalized 1,2,3,4-Tetrahydropyridines by the Reaction of 3-[(Propan-2-ylsulfanyl)methyl]pentane-2,4-dione with Anilines
IF 0.9
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A method for the synthesis of 1,1,1-(6-methyl-1-aryl-1,2,3,4-tetrahydropyridine-3,3,5-triyl)triethanones by the reaction of 3-[(propan-2-ylsulfanyl)methyl]pentane-2,4-dione with anilines in the presence of catalytic amounts of acetic acid was developed. It was found that the reactivity of substituted anilines in the studied reaction depends on the nature and position of the substituent, decreasing in the series: 2,4-Me2-С6H3NH2 > С6H5NH2 4-Me-С6H4NH2 2-Me-С6H4NH2 > 3-Me-С6H4NH2 2-MeO-С6H4NH2 > 4-MeC(O)-С6H4NH2 4-Br-С6H4NH2.
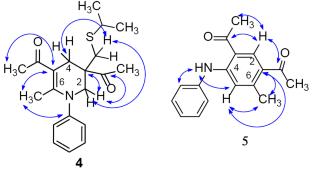
3-[(丙-2-基磺胺基)甲基]戊烷-2,4-二酮与苯胺反应合成功能化1,2,3,4-四氢吡啶
以3-[(丙基-2-酰基磺酰)甲基]戊烷-2,4-二酮与苯胺为原料,在乙酸的催化量下合成1,1,1-(6-甲基-1-芳基-1,2,3,4-四氢吡啶-3,3,5-三基)三乙烷酮。研究发现,取代苯胺的反应活性取决于取代基的性质和位置,其级数依次降低:2,4- me2 -С6H3NH2 >; С6H5NH2 4-Me-С6H4NH2 2- me -С6H4NH2 > 3-Me-С6H4NH2 2- meo -С6H4NH2 > 4-MeC(O)-С6H4NH2 4-Br-С6H4NH2。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


