Flower-like Fe3O4@manganese dioxide@tannic acid core-shell structure for efficient adsorption of dyes and Mn (VII) in water
IF 5.2
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
It causes water pollution mainly from textile wastewater and industrial wastewater in large quantities, which causes irreversible harm to the environment. In this study, a novel composite adsorbent (Fe3O4@MnO2@TA) was synthesized by integrating tannic acid (TA) with Fe3O4 and MnO2 via a hydrothermal method. The adsorption performance of the composite toward methylene blue (MB), malachite green (MG), crystal violet (CV), and Mn (VII) in aqueous solution was systematically investigated. The Fe3O4@MnO2@TA composites were characterized by SEM, TEM, XRD, FTIR, TGA and BET. The magnetic magnitude of Fe3O4@MnO2@TA composites was 5.06 emu g−1, which provided a magnetic basis for the recovery of the composites. Considering the target range of various influencing factors during the adsorption process, the effects of such variables as adsorbent type, initial concentration, adsorption time, and adsorption temperature on pollutant adsorption efficiency were investigated. The adsorption behavior of MB, MG, CV, and Mn (VII) was analyzed using several kinetic and isotherm models, with the Fe3O4@MnO2@TA composites satisfied the pseudo-second-order kinetic model and Langmuir isotherm models. The interactions existing between the conformal materials and MB, MG, CV and Mn (VII) were simulated by Density Functional Theory (DFT) calculations, and the results of the simulations can be matched with the experimental data. The adsorption mechanisms are mainly related to electrostatic interactions, n-π/π-π interactions, and hydrogen bonding interactions. In conclusion, Fe3O4@MnO2@TA composites are adsorbents with potential applications for the removal of ions and dyes from wastewater.
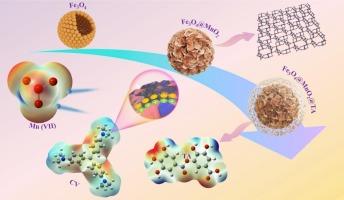
花状Fe3O4@manganese dioxide@tannic酸核壳结构,有效吸附水中染料和Mn (VII)
它造成的水污染主要来自于大量的纺织废水和工业废水,对环境造成了不可逆转的危害。本研究通过水热法将单宁酸(TA)与Fe3O4和MnO2结合,合成了一种新型复合吸附剂Fe3O4@MnO2@TA。研究了该复合材料对亚甲基蓝(MB)、孔雀石绿(MG)、结晶紫(CV)和锰(VII)的吸附性能。通过SEM、TEM、XRD、FTIR、TGA和BET对Fe3O4@MnO2@TA复合材料进行了表征。Fe3O4@MnO2@TA复合材料的磁性强度为5.06 emu g−1,为复合材料的回收提供了磁性基础。考虑到吸附过程中各种影响因素的目标范围,考察了吸附剂类型、初始浓度、吸附时间、吸附温度等变量对污染物吸附效率的影响。采用多种动力学模型和等温线模型对MB、MG、CV和Mn (VII)的吸附行为进行了分析,Fe3O4@MnO2@TA复合材料满足拟二级动力学模型和Langmuir等温线模型。利用密度泛函理论(DFT)模拟了共形材料与MB、MG、CV和Mn (VII)之间的相互作用,模拟结果与实验数据吻合较好。吸附机理主要与静电相互作用、n-π/π-π相互作用和氢键相互作用有关。综上所述,Fe3O4@MnO2@TA复合材料在去除废水中的离子和染料方面具有潜在的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


