Non-linear tricationic ionic liquid (NLTCIL) as a novel CO₂ absorbent: Investigating the effect of water with a dual MD/COSMO-RS approach
IF 5.2
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
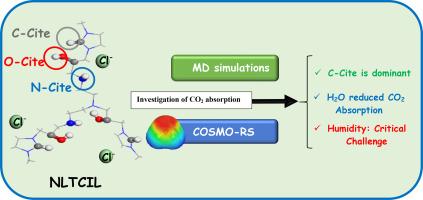
This study leverages MD simulations and COSMO-RS analysis to thoroughly examine a nonlinear tricationic ionic liquid (NLTCIL) containing amine and hydroxyl functional groups, elucidating its intricate interactions with water and CO2. MD simulations reliably predicted physical properties, showing water decreases density while CO2 increases it. Microscopic structural analysis, through RDF, SDF, and CDF, revealed that while the strongest interactions in pure NLTCIL involve chloride anions and imidazolium ring hydrogens, water significantly disrupts these, forming extensive hydrogen bond networks and drastically reducing hydrogen bond lifetimes. CO2, in contrast, exhibited a minor structural impact, primarily occupying voids. 3D RDG distributions highlighted dominant van der Waals interactions around CO2, with water increasing weaker, non-specific interactions and reducing strong hydrogen bonds. TFI visualizations depicted stable CO2 interactions in pure NLTCIL (green/blue regions) but less stable, fluctuating ones (red regions) in water-containing systems, lowering CO2 absorption capacity. Sigma profiles and chemical potential overlaps revealed water's high polarity and strong hydrogen bonding with NLTCIL, outcompeting weaker CO2 interactions, explaining CO2's low solubility in water and humidity's negative impact on absorption. COSMO-RS analysis highlighted NLTCIL's potential for industrial gas separation due to significant CO2 solubility enhancement (up to tenfold at 10 bar) and high selectivity. However, humidity emerged as a critical challenge. The absorption mechanism was primarily linked to interactions at the N, O, and C sites of the cation, with the C-site potentially playing a more significant role. These findings provide crucial groundwork for designing humidity-resistant ionic liquids and optimizing CO2 absorption processes.
非线性三阳离子离子液体(NLTCIL)作为一种新型的CO₂吸附剂:用双MD/ cosmos - rs方法研究水的作用
本研究利用MD模拟和cosmos - rs分析彻底研究了一种含有胺和羟基官能团的非线性三阳离子离子液体(NLTCIL),阐明了其与水和二氧化碳的复杂相互作用。MD模拟可靠地预测了物理性质,表明水降低密度而二氧化碳增加密度。通过RDF、SDF和CDF进行的微观结构分析显示,尽管纯NLTCIL中最强的相互作用涉及氯阴离子和咪唑环氢,但水显著破坏了这些相互作用,形成了广泛的氢键网络,并大大缩短了氢键寿命。相比之下,二氧化碳对结构的影响较小,主要占据空隙。3D RDG分布突出了CO2周围主要的范德华相互作用,水增加了较弱的非特异性相互作用,减少了强氢键。TFI可视化描述了纯NLTCIL中稳定的CO2相互作用(绿色/蓝色区域),但在含水系统中不太稳定,波动的相互作用(红色区域),降低了CO2吸收能力。Sigma剖面和化学势重叠揭示了水与NLTCIL的高极性和强氢键,胜过了较弱的CO2相互作用,解释了CO2在水中的低溶解度和湿度对吸收的负面影响。COSMO-RS分析强调了NLTCIL在工业气体分离方面的潜力,因为它显著提高了二氧化碳的溶解度(在10 bar时高达10倍)和高选择性。然而,湿度成为了一个关键的挑战。吸附机制主要与阳离子的N、O和C位点的相互作用有关,其中C位点可能起着更重要的作用。这些发现为设计抗湿离子液体和优化二氧化碳吸收过程提供了重要的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


