Solar light driven Zn@CuO/MoO3 heterojunction photocatalyst for photodegradation of methylene blue dye: Enhanced stability, charge separation, and environmental remediation
IF 5.2
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
A novel Zn@CuO/MoO3 binary heterojunction photocatalyst was synthesized via a wet chemical route and investigated for the degradation of methylene blue (MB) under visible light. XRD confirmed a monoclinic structure with ⁓20 nm crystallite size, while FTIR, Raman, TGA-DSC, and XPS analyses verified strong interfacial interaction, stability, and the presence of key oxidation states. SEM-EDX and elemental mapping revealed a uniform distribution of nanoparticles with the expected composition. UV–visible and PL showed enhanced optical absorption and reduced charge recombination. The Mott-Schottky curve of Zn@CuO/MoO3 showed a positive slope, indicating n-type semiconductor behavior. Under optimal conditions, Zn@CuO/MoO3 achieved 100 % photodegradation of MB in 30 min, demonstrating superior performance compared to pristine CuO, MoO3, and Zn@CuO, which maintained 97 % efficiency after four cycles. Radical trapping tests revealed that hydroxyl [·OH] and superoxide [·O2−] radicals played dominant roles in the degradation process, and the degradation followed pseudo-second-order kinetics with a rate constant of 0.10 g mg−1 min−1 (R2 = 0.999). GC–MS and ECOSAR confirmed the reduced toxicity of byproducts. The superior performance of Zn@CuO/MoO3 is attributed to enhanced UV–visible absorption, efficient charge separation, and reduced electron-hole recombination, highlighting its potential as a cost-effective, reusable, and efficient p-n heterojunction photocatalyst for environmental applications.
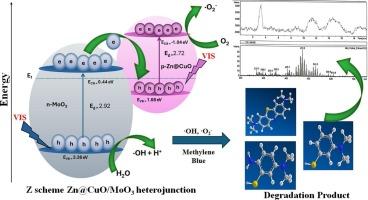
太阳能光驱动Zn@CuO/MoO3异质结光催化剂光降解亚甲基蓝染料:增强稳定性、电荷分离和环境修复
采用湿法合成了一种新型Zn@CuO/MoO3二元异质结光催化剂,并对其在可见光下对亚甲基蓝(MB)的降解进行了研究。XRD证实为单斜斜结构,晶体尺寸为⁓20 nm, FTIR、Raman、TGA-DSC和XPS分析证实了强的界面相互作用、稳定性和关键氧化态的存在。SEM-EDX和元素映射显示纳米颗粒的均匀分布与预期的组成。紫外可见和PL表现出增强的光吸收和减少的电荷复合。Zn@CuO/MoO3的Mott-Schottky曲线斜率为正,表现为n型半导体行为。在最佳条件下,Zn@CuO/MoO3在30分钟内实现了100%的MB光降解,与原始CuO、MoO3和Zn@CuO相比,表现出了卓越的性能,后者在四个循环后仍保持97%的效率。自由基捕获试验表明,羟基[·OH]和超氧化物[·O2−]自由基在降解过程中起主导作用,降解过程符合准二级动力学,速率常数为0.10 g mg−1 min−1 (R2 = 0.999)。GC-MS和ECOSAR证实副产物毒性降低。Zn@CuO/MoO3的优异性能归功于增强的紫外可见吸收,高效的电荷分离和减少的电子-空穴复合,突出了其作为一种具有成本效益,可重复使用和高效的环境应用p-n异质结光催化剂的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


