Nature-Inspired Glycosylation Strategy Enabled Hydrosoluble Polyhydric Thioalkylated Ferrocene Derivatives for pH-Neutral Aqueous Redox Flow Batteries
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Organic molecules have been regarded as promising alternatives in aqueous redox flow batteries, with the aim of reducing reliance on mineral resources. Enhancing the solubility and stability of organic species is essential and requires strategic functional group refinement and molecular structure optimization. However, there are relatively few solubilization strategies of naturally water-solubilizing groups in ARFBs. Sugars, i.e., carbohydrates, ubiquitous in nature and indispensable as nutrients, possess an exceptional hydrophilic property and offer a sustainable pathway for molecular functionalization. Herein, we present a thioglucose functionalization strategy to synthesize highly soluble ferrocene derivatives via convenient thioetherification reactions under mild conditions. Under the hydrophilic effect of abundant highly polar hydroxyl moieties, the as-synthesized glycosyl-functionalized thioalkylated ferrocene derivative, namely, Fc-(Thio-Glc)2, exhibits high water solubility (1.3 M in 1.0 M KCl solution) and favorable electrochemical properties. Molecular dynamics simulations manifest the effects of hydrogen bond networks on the molecular configuration and solvation behavior. Ex situ spectroscopic analyses confirmed the high reversibility and long-term operation stability of Fc(Thio-Glc)2. Consequently, the pH-neutral ARFBs assembled with the 0.5 M Fc(Thio-Glc)2 catholyte achieve a capacity retention of 99.995% per cycle or 99.82% per day. This study highlights the tremendous potential of a bioinspired molecular engineering strategy in advancing safe, stable, and sustainable ARFBs toward large-scale energy storage applications.
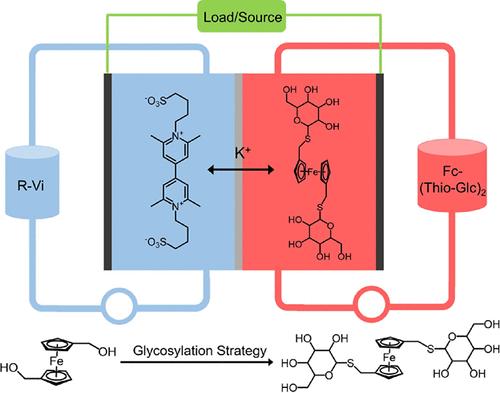
受自然启发的糖基化策略使ph中性水氧化还原液流电池的水溶性多氢硫烷基化二茂铁衍生物成为可能
有机分子被认为是水氧化还原液流电池中有前途的替代品,其目的是减少对矿物资源的依赖。提高有机物种的溶解度和稳定性至关重要,这需要战略性的官能团精炼和分子结构优化。然而,arfb中天然水溶性基团的增溶策略相对较少。糖,即碳水化合物,在自然界中无处不在,是必不可少的营养物质,具有特殊的亲水性,并为分子功能化提供了可持续的途径。在此,我们提出了一种巯基葡萄糖功能化策略,在温和的条件下通过方便的硫醚化反应合成高可溶性二茂铁衍生物。在大量高极性羟基基团的亲水性作用下,合成的糖基功能化硫代烷基化二茂铁衍生物Fc-(Thio-Glc)2在1.0 M KCl溶液中具有较高的水溶性(1.3 M)和良好的电化学性能。分子动力学模拟显示了氢键网络对分子构型和溶剂化行为的影响。非原位光谱分析证实了Fc(Thio-Glc)2的高可逆性和长期运行稳定性。因此,与0.5 M Fc(Thio-Glc)2阴极电解质组装的ph中性arfb每个循环的容量保持率为99.995%或每天99.82%。这项研究强调了生物启发的分子工程策略在推进安全、稳定和可持续的arfb向大规模储能应用方面的巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


