Cation Competition Experiments during Electrochemical CO2 Reduction As a Probe of the Film-Modified Copper Microenvironment
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Alkali metal electrolyte cations affect product activity and selectivity in electrochemical CO2 reduction (CO2R); however, the precise mechanisms of action are not fully understood. By performing cation competition experiments with LiHCO3 and CsHCO3 during CO2R with bare and an organic film-modified Cu electrode, we deconvolute cation-dependent electric-field and nonelectric-field effects and their impact on CO2R performance. Attenuated total reflection surface-enhanced infrared absorption spectroscopy (ATR-SEIRAS) experiments reveal that organic additive films do not change cation accumulation at the electrochemical interface. Instead, through time-resolved ATR-SEIRAS experiments, we find that additive films alter the transport and interfacial concentrations of CO2 and CO, a CO2R intermediate. This lowers the required [Cs+] to achieve improved CO2R without changing the intrinsic reaction kinetics. Additionally, we find that even small amounts of Cs+ significantly disrupt the interfacial water structure, which we infer is key to the promotion of CO2R to CO and/or CO reduction to C–C coupled products. Together, this study yields spectroscopic evidence for the mechanism of improved CO2R selectivity with organic film-modified electrodes and decouples electric-field and nonelectric-field cation effects in CO2R.
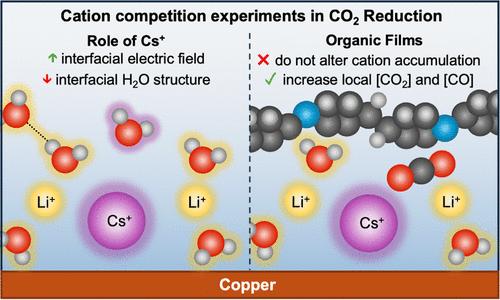
电化学CO2还原过程中的阳离子竞争实验——膜修饰铜微环境的探针
碱金属电解质阳离子对电化学CO2还原产物活性和选择性的影响然而,确切的作用机制尚不完全清楚。通过在裸铜电极和有机膜修饰铜电极上进行CO2R过程中与LiHCO3和CsHCO3的阳离子竞争实验,研究了阳离子依赖的电场和非电场效应及其对CO2R性能的影响。衰减全反射表面增强红外吸收光谱(ATR-SEIRAS)实验表明,有机添加剂膜不会改变电化学界面处阳离子的积累。相反,通过时间分辨ATR-SEIRAS实验,我们发现添加剂薄膜改变了CO2和CO(一种CO2R中间体)的传输和界面浓度。这降低了在不改变固有反应动力学的情况下实现改善CO2R所需的[Cs+]。此外,我们发现即使少量的Cs+也会显著破坏界面水结构,我们推断这是促进CO2R转化为CO和/或CO还原为C-C偶联产物的关键。总之,本研究为有机膜修饰电极提高CO2R选择性的机理提供了光谱证据,并解耦了CO2R中的电场和非电场阳离子效应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


