Cations Enhance Hydride Transfer to Noncatalytic Metals in Concentrated Alkaline Electrolytes
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Alkali metal cations are known to influence the kinetics of the hydrogen evolution reaction (HER), acting as either promoters or inhibitors to the rate-determining step depending on the metal surface, local pH, and cation concentration. Despite the importance of concentrated electrolytes for commercial electrochemical cells, the impact of cations on the HER in concentrated alkaline environments (>1 M) and in mixed cation systems remains poorly understood. This study quantifies the HER kinetics at polycrystalline metal surfaces (Pt, Au, Cu, and Fe) and the equilibrium solvation environment in pure and mixed alkali metal hydroxide electrolytes at concentrations up to 3.0 M. Kinetic analyses of Au, Cu, and Fe revealed a positive cation-concentration-effect that was primarily driven by changes to the charge transfer coefficient. Multinuclear NMR spectroscopy examined the solvation of H2O/OH– species and the alkali cations as a function of (mixed) alkali cation concentration(s), and demonstrated rapid exchange between solvent, hydroxide, and solvated cations. Together, these findings support models where HER kinetics on noncatalytic metal surfaces in strongly alkaline conditions are primarily governed by the average polarization and polarizability of the metal/solution interface and that increasing cation activity continues to increase the transfer coefficient at metal-hydroxide concentrations up to 3.0 M. Electrolytes and additives which can outcompete weakly hydrated cations and disrupt the interfacial water structure are expected to suppress parasitic HER at electrodes for energy storage and electroplating.
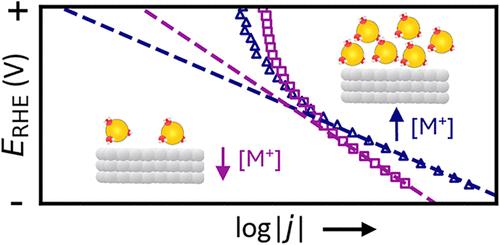
在浓碱性电解质中,阳离子促进氢化物向非催化金属的转移
已知碱金属阳离子会影响析氢反应(HER)的动力学,根据金属表面、局部pH和阳离子浓度,在速率决定步骤中起到促进剂或抑制剂的作用。尽管浓缩电解质对商用电化学电池很重要,但在浓碱性环境(1 M)和混合阳离子体系中,阳离子对HER的影响仍然知之甚少。本研究量化了多晶金属表面(Pt、Au、Cu和Fe)的HER动力学,以及浓度高达3.0 m的纯和混合碱金属氢氧化物电解质的平衡溶剂化环境。对Au、Cu和Fe的动力学分析显示,阳离子浓度效应主要由电荷传递系数的变化驱动。多核核磁共振波谱检测了H2O/OH -的溶剂化和碱阳离子作为(混合)碱阳离子浓度的函数,并证明了溶剂、氢氧化物和溶剂化阳离子之间的快速交换。在一起,这些发现支持了一些模型,即在强碱性条件下,非催化金属表面上的HER动力学主要由金属/溶液界面的平均极化和极化率控制,并且在金属氢氧化物浓度达到3.0 m时,阳离子活度的增加继续增加传递系数,电解质和添加剂可以比弱水合阳离子竞争并破坏界面水结构,有望抑制寄生HER用于储能和电镀的电极。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


