Deaminative Functionalization of Olefins and Dienes with Katritzky Salts: A Highly Selective Route to Diverse Deuterium-Labeled Molecules
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, we report a deaminative alkylation of olefins, followed by deuteration that allows the formation of sp3–sp3 architectures. This protocol enables the previously unexploited functionalization of dienes with pyridinium salts, providing rapid access to complex molecules from bulk chemicals in a highly selective manner. Notably, the diversification of commercially available drug molecules particularly through deuteration is widely recognized as an effective strategy for drug discovery. Gratifyingly, this reaction facilitated the alkylation of drug- and amino acid-derived pyridinium salts, demonstrating both its synthetic versatility and potential utility in drug development.
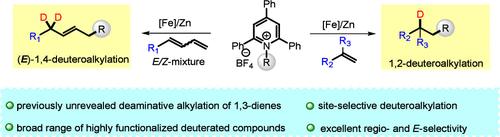
烯烃和二烯与Katritzky盐的脱胺功能化:一个高度选择性的途径不同的氘标记分子。
在这里,我们报道了烯烃的脱胺烷基化,然后是氘化,允许形成sp3-sp3结构。该方案使以前未开发的二烯与吡啶盐的功能化成为可能,以高度选择性的方式从散装化学品中快速获取复杂分子。值得注意的是,商业上可获得的药物分子的多样化,特别是通过氘化,被广泛认为是一种有效的药物发现策略。令人欣慰的是,该反应促进了药物和氨基酸衍生的吡啶盐的烷基化,证明了其合成的多功能性和在药物开发中的潜在用途。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


