Charge-Transfer States Enable Spin-Selective Formation of Quartet State Qudits in Luminescent Radical-Chromophore Dyads
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Luminescent tris(2,4,6-trichlorophenyl)methyl (TTM) radicals are promising doublet emitters that have been used to generate spin-optical interfaces in molecular electron spin qudits. In particular, photoexcitation of covalent TTM-chromophore dyads can generate quartet spin states that can be detected via photoluminescence. However, the mechanism of quartet spin initialization is complicated by competing charge-transfer (CT) and energy-transfer (EnT) pathways between the radical and the chromophore. Here, we demonstrate the role of CT intermediates by engineering a covalent dyad of TTM and naphthalene-(1,4:5,8)-bis(dicarboximide) (NDI), a well-known electron acceptor chromophore. Transient absorption spectroscopy indicates that photoexcitation of TTM results in ultrafast electron transfer from 2*TTM to NDI, which kinetically outcompetes EnT. Electron paramagnetic resonance spectroscopy reveals that charge recombination proceeds via spin–orbit charge-transfer intersystem crossing to generate 3*NDI, followed by spin mixing with 2TTM to form the quartet state. This process uniquely spin-polarizes the quartet state, providing fundamental design principles to purify the initial wave function for quantum information processing.
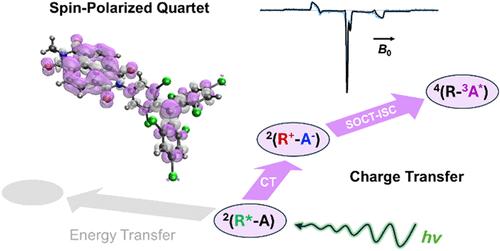
发光自由基-发色团二元体中电荷转移态使自旋选择性形成四重奏态量子
发光三(2,4,6-三氯苯基)甲基(TTM)自由基是一种很有前途的双重发射体,已被用于在分子电子自旋量子态中产生自旋光学界面。特别是,共价ttm -发色团二偶体的光激发可以产生可以通过光致发光检测到的四重奏自旋态。然而,由于自由基和发色团之间相互竞争的电荷转移(CT)和能量转移(EnT)途径,使得四重奏自旋初始化的机制变得复杂。在这里,我们通过设计一个TTM和萘-(1,4:5,8)-双(二碳酰亚胺)(NDI)的共价二偶体来证明CT中间体的作用,萘-(1,4:5,8)-二(二碳酰亚胺)(NDI)是一种众所周知的电子受体发色团。瞬态吸收光谱表明,TTM的光激发导致2*TTM向NDI的超快电子转移,在动力学上优于EnT。电子顺磁共振波谱显示,电荷复合是通过自旋轨道电荷转移系统间交叉产生3*NDI,然后与2TTM自旋混合形成四重态。该过程独特地自旋极化了四重奏态,为净化量子信息处理的初始波函数提供了基本的设计原理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


