Inhibiting Isomerization via Confinement in Ice–Water Interfaces Enhances Stereoselectivity in [2 + 2] Cycloaddition
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Cyclobutanes serve as fundamental motifs in natural products, bioactive substrates, and pharmaceuticals. Despite numerous efforts to synthesize cyclobutane products, particularly through light-driven [2 + 2] cycloaddition, significant challenges persist, such as the rapid cis/trans isomerization of olefin substrates and the thermodynamic difficulty in synthesizing syn-cyclobutane derivatives, which limit their widespread application across various fields. Here, by introducing a confined ice–water interface to mediate the [2 + 2] photocycloadditions, we not only efficiently inhibit reactant isomerization but also achieve the syn-cyclobutane products (syn-dimer > 95%) without the need for additional chemical auxiliary reagents. The specific proportional relationship of reaction kinetics and the active ice surface area reveals the unique catalytic role of the ice surface in [2 + 2] cycloaddition reactions. Theoretical simulations and experimental studies demonstrate that at the ice–water interface, solute molecules tend to adsorb on the ice surface and form molecular pairs in a prereactive arrangement. Compared to molecular monomers, these molecular pairs exhibit a higher energy barrier for photoisomerization and tend to align in a syn-configuration, thereby inhibiting isomerization and facilitating stereoselective [2 + 2] cycloaddition. This work highlights the potential of ice-assisted photochemical methods in advancing sustainable and environmentally friendly chemical synthesis.
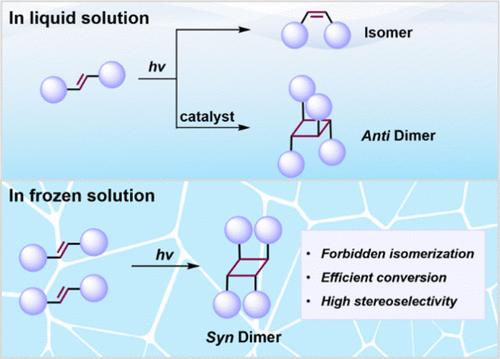
通过限制冰水界面抑制异构化提高[2 + 2]环加成的立体选择性
环丁烷是天然产物、生物活性底物和药物的基本基序。尽管在合成环丁烷产品方面做出了许多努力,特别是通过光驱动[2 + 2]环加成,但仍然存在重大挑战,例如烯烃底物的快速顺/反异构化以及合成顺-环丁烷衍生物的热力学困难,这限制了它们在各个领域的广泛应用。在这里,通过引入一个受限的冰-水界面来介导[2 + 2]光环加成,我们不仅有效地抑制了反应物的异构化,而且在不需要额外的化学辅助试剂的情况下获得了同型环丁烷产物(同型二聚体>; 95%)。反应动力学与活性冰表面积的比例关系揭示了冰表面在[2 + 2]环加成反应中的独特催化作用。理论模拟和实验研究表明,在冰-水界面处,溶质分子倾向于吸附在冰表面,并形成分子对以预反应的方式排列。与分子单体相比,这些分子对具有更高的光异构能垒,并倾向于以同步构型排列,从而抑制异构化并促进立体选择性[2 + 2]环加成。这项工作强调了冰辅助光化学方法在促进可持续和环境友好的化学合成方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


