Structure-Guided Design of a Bioactive Covalent Small Molecule Targeting a Riboswitch
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Small molecule ligands targeting structured RNA elements hold promise for modulating RNA function, serving as chemical probes and potential therapeutics. In this study, the characterization of phenylglyoxal-based covalent probe designed to target unpaired guanine residues in structured RNAs is reported. A structure-guided design strategy was employed to modify covalently unpaired guanines critical for flavin mononucleotide (FMN) binding to the FMN riboswitch. Covalent modification occurs at the designed site and modulates riboswitch function in a cellular reporter system, highlighting the potential of covalent mechanisms of action for bioactive RNA ligands.
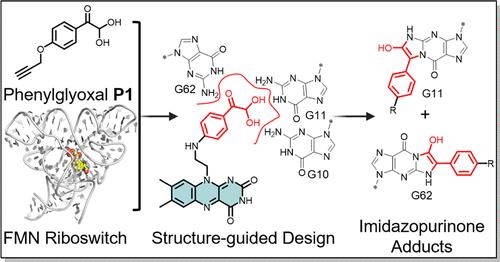
靶向核糖开关的生物活性共价小分子的结构引导设计
靶向结构RNA元件的小分子配体有望调节RNA功能,作为化学探针和潜在的治疗方法。在这项研究中,以苯基乙二醛为基础的共价探针被设计用于靶向结构rna中未配对的鸟嘌呤残基。采用结构导向设计策略修饰共价未配对的鸟嘌呤,这些鸟嘌呤是黄素单核苷酸(FMN)与FMN核糖开关结合的关键。共价修饰发生在设计位点并调节细胞报告系统中的核糖开关功能,突出了生物活性RNA配体的共价作用机制的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


