Stapling of β-Glucans Increases Antibody Binding
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Higher-order structures are essential for the function of biological macromolecules. Tuning the conformational space of peptides by stapling improves their pharmacological properties. The three-dimensional (3D) structures of glycans are much less well understood than those of peptides and oligonucleotides, and willful modulation of oligosaccharide structures to improve binding to proteins has not been described to date. Herein, we describe stapling of β-(1,3)-glucans to tune their conformation, aiming to mimick the naturally occurring triple helix. The stapled glycans are prepared by automated glycan assembly, followed by linker construction and ring-closure assisted by solid-phase peptide synthesis. Thereby, staples of different lengths, polarities, and topologies can be readily introduced. Molecular dynamics simulations served to evaluate the effect of stapling on the conformational space. Glycan microarray experiments revealed that stapled glycans bound significantly more tightly to monoclonal mouse and rabbit antibodies than did linear glycans. Controlling the conformational space of short oligosaccharides creates opportunities for synthetic glycans in drug and vaccine development.
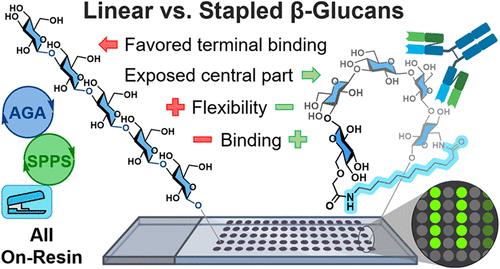
β-葡聚糖的钉接增加抗体结合
高阶结构对生物大分子的功能至关重要。通过钉接调整肽的构象空间可以改善其药理特性。聚糖的三维(3D)结构比肽和寡核苷酸的三维(3D)结构了解得少得多,并且有意调节寡糖结构以改善与蛋白质的结合至今尚未描述。在这里,我们描述了β-(1,3)-葡聚糖的钉接,以调整其构象,旨在模仿自然发生的三螺旋结构。通过自动组装聚糖,然后在固相肽合成的辅助下进行连接剂构建和环闭合,制备了钉接聚糖。因此,可以很容易地引入不同长度、极性和拓扑结构的订书钉。分子动力学模拟用于评价钉接对构象空间的影响。聚糖微阵列实验显示,与线性聚糖相比,钉接聚糖与单克隆小鼠和兔抗体的结合明显更紧密。控制短寡糖的构象空间为药物和疫苗开发中的合成聚糖创造了机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


