Ion-Specific Interfacial Electric Fields on Water Microdroplets for Tuning Menshutkin Reactions
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
High interfacial electric fields (EFs) on water microdroplets can drastically accelerate chemical reactions; however, how dissolved ions can alter these fields and associated reaction kinetics remain elusive. By combining microdroplet experiments, molecular dynamics (MD) simulations, and quantum chemical calculations, here we studied the ion-specific effects in the pyridine–iodomethane Menshutkin reaction, a reaction that can be accelerated by the EF at the air–water interface of microdroplets. Experimental results reveal that monovalent anions regulate reaction yields in accordance with the Hofmeister series (HCOO– > F– > Cl– > NO3– > BF4– ≈ SCN– > ClO4– > PF6–). Theoretical analyses show that the strength of the EF depends on the interplay between the interfacial affinity of ions and hydration energy: cations with high interfacial affinity and strong hydration capacity amplify interfacial EFs and accelerate reaction rates, whereas anions adsorb to the interface competitively, weakening EFs and suppressing reactivity. This work deciphers how ion-specific interfacial microenvironments govern chemical reactivity in microdroplet systems, providing a basis for the rational design of new EF accelerated microdroplet reactions.
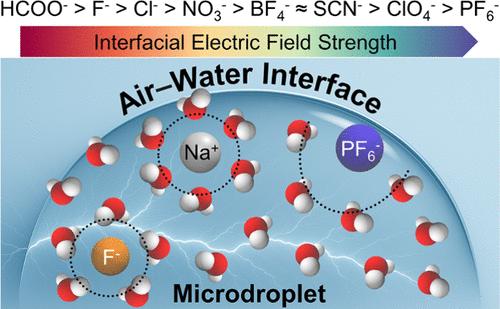
微水滴上调节Menshutkin反应的离子特异性界面电场
水微滴上的高界面电场能显著加速化学反应;然而,溶解的离子如何改变这些场和相关的反应动力学仍然是难以捉摸的。本文采用微滴实验、分子动力学模拟和量子化学计算相结合的方法,研究了微滴空气-水界面处EF加速的吡啶-碘甲烷Menshutkin反应中的离子特异性效应。实验结果表明,单价阴离子按照Hofmeister级数(HCOO - > F - > Cl - > NO3 - > BF4 -≈SCN - > ClO4 - > PF6 -)调节反应产率。理论分析表明,界面电场的强度取决于离子的界面亲和力和水化能之间的相互作用:界面亲和力高、水化能力强的阳离子会放大界面电场,加快反应速度,而阴离子则会竞争性地吸附在界面上,削弱电场,抑制反应活性。这项工作揭示了离子特异性界面微环境如何控制微滴系统中的化学反应性,为合理设计新型EF加速微滴反应提供了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


