Neurensin-2 is a negative regulator of CB1 receptor
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Major depressive disorder (MDD) is a leading cause of disability worldwide. Unfortunately, approximately 30 % of MDD patients do not respond to available antidepressants, and characterization of novel molecular mediators of MDD is urgently needed. Chronic stress, a key risk factor for MDD, induces widespread neurobiological changes, including alterations in synaptic plasticity and neurotransmission. However, the molecular mechanisms mediating these effects remain poorly defined.
In this study, we provide evidence that Neurensin-2, a stress-inducible vesicular protein, regulates the cannabinoid receptor 1 (CB1R), an important mediator of depression. In N2a cells, overexpression of Neurensin-2 was associated with reduced CB1R expression and attenuation of its downstream signaling; the Akt, mTOR, and Erk1/2 pathways. In these cells, Neurensin-2 co-localized with CB1R and reduced the expression of this receptor in both the membrane and intracellular organelles. The Neurensin-2-CB1R relationship was examined in vivo using the chronic social defeat stress (CSDS) model, where stress exposure was accompanied by increased Nrsn2 and decreased Cnr1 expression in the mouse hippocampus. Notably, stress-induced Cnr1 downregulation was not observed in Neurensin-2 knockout mice, suggesting that Neurensin-2 plays a role in stress-induced downregulation of CB1R in vivo. We further confirmed these expression patterns within the hippocampal inhibitory neuronal population that highly co-expresses Neurensin-2 and CB1R, the CCK-positive hippocampal interneurons.
These findings uncover a novel stress-induced pathway that inhibits CB1R signaling by Neurensin-2. Thus, we suggest that Neurensin-2 is a potential therapeutic target for treatment-resistant depression.
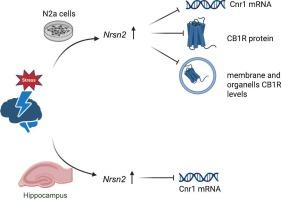
神经素-2是CB1受体的负调节因子。
重度抑郁症(MDD)是世界范围内致残的主要原因。不幸的是,大约30% %的MDD患者对现有的抗抑郁药物没有反应,迫切需要对MDD的新型分子介质进行表征。慢性应激是重度抑郁症的一个关键危险因素,可引起广泛的神经生物学变化,包括突触可塑性和神经传递的改变。然而,介导这些效应的分子机制仍然不明确。在这项研究中,我们提供的证据表明,神经素-2,应激诱导的囊泡蛋白,调节大麻素受体1 (CB1R),抑郁症的重要介质。在N2a细胞中,Neurensin-2的过表达与CB1R表达减少及其下游信号通路Akt、mTOR和ERK1/2的衰减有关。在这些细胞中,神经球蛋白-2与CB1R共定位,并降低了该受体在膜和胞内细胞器中的表达。使用慢性社会失败应激(CSDS)模型在体内研究了神经球蛋白-2- cb1r的关系,其中应激暴露伴随着小鼠海马Nrsn2的增加和Cnr1表达的降低。值得注意的是,在Neurensin-2敲除小鼠中未观察到应激诱导的CB1R下调,这表明Neurensin-2在体内应激诱导的CB1R下调中起作用。我们进一步证实了这些在海马抑制性神经元群体中的表达模式,这些神经元群体高度共同表达Neurensin-2和CB1R, cck阳性海马中间神经元。这些发现揭示了一种新的应激诱导途径,可抑制神经素-2的CB1R信号传导。因此,我们认为神经素-2是治疗难治性抑郁症的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


