Genetic variants of cytochrome P450 2C21 identified by screening 6344 dogs influenced oxidations of the probe drug omeprazole
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The cytochromes P450 (P450s or CYPs) are essential drug-metabolizing enzymes. In humans, the variability in metabolic activity is partly accounted for by genetic variants of the responsible P450. However, P450 genetic variants remain largely to be investigated in dogs, a species often used in drug metabolism studies. In this study, the sequencing of 6344 dog genomes found five CYP2C21 variants, including three missense variants (p.K119Q, p.R125C, and p.R329H), one synonymous variant, and one frameshift variant (c.669dupA). The latter creates a premature termination codon that results in the elimination of the heme-binding region and substrate recognition sites 3–6; these are important functional domains, and thus c.669dupA is considered to be a null allele. Some of these variants were found only in a limited number of dogs and breeds, indicating that their distribution may be restricted. Recombinant proteins of the missense variants, co-expressed with NADPH-P450 reductase, were prepared in Escherichia coli for metabolic assays. The CYP2C21 R125C variant showed decreased metabolic capacity compared with the wild-type CYP2C21 protein. These results suggest the possible contribution of genetic variants of CYP2C21 to the variability of drug metabolism in dogs.
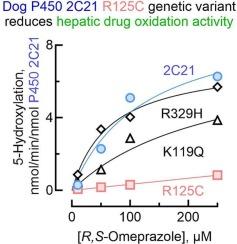
通过筛选6344只狗,发现细胞色素P450 2C21基因变异影响探针药物奥美拉唑的氧化作用。
细胞色素P450 (P450或CYPs)是必不可少的药物代谢酶。在人类中,代谢活动的可变性部分是由P450的遗传变异引起的。然而,P450基因变异在很大程度上仍有待研究,狗是一种经常用于药物代谢研究的物种。本研究对6344个犬基因组进行测序,发现5个CYP2C21变异,包括3个错义变异(p.K119Q、p.R125C和p.R329H)和1个移码变异(c.r 669dupa)。后者产生一个过早终止密码子,导致血红素结合区和底物识别位点3-6的消除;这些是重要的功能域,因此c.669dupA被认为是一个空等位基因。其中一些变异只在有限数量的狗和品种中发现,这表明它们的分布可能受到限制。在大肠杆菌中制备了与NADPH-P450还原酶共表达的错义变异体重组蛋白,用于代谢分析。CYP2C21 R125C变异与野生型CYP2C21蛋白相比,代谢能力下降。这些结果表明CYP2C21基因变异可能对狗的药物代谢变异性有贡献。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


