VIRMA-mediated SHQ1 m6A modification enhances liver regeneration through an HNRNPA2B1-dependent mechanism
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
N6-Methyladenosine (m6A) modification is a crucial post-transcriptional regulatory mechanism and the most abundant and highly conserved RNA epigenetic modification in eukaryotes. Previous studies have indicated the involvement of m6A modification in various tissue regeneration processes, including liver regeneration. Vir-like m6A methyltransferase associated protein (VIRMA) is an m6A methyltransferase with robust methylation capability. However, its role in liver regeneration remains poorly understood. In this study, we generated liver-specific Virma knockout mice using the Cre-loxP system and investigated the biological functions of VIRMA in liver regeneration using both the Associating Liver Partition and Portal vein Ligation for Staged Hepatectomy (ALPPS) mouse model and the carbon tetrachloride (CCl4) mouse model. The expression level of VIRMA was rapidly up-regulated after ALPPS surgery and gradually down-regulated during liver repair. Virma deficiency significantly impaired liver regeneration capacity and disrupted cell cycle progression. Methylated RNA immunoprecipitation sequencing (MeRIP-seq) analysis revealed that Shq1 is an effective downstream target of VIRMA-mediated m6A modification. The upregulation of Shq1 enhanced the proliferation ability of cells, which was attenuated by the specific AKT inhibitor ipatasertib. Supplementation of Shq1 in vivo alleviated the liver cell proliferation inhibition caused by Virma deficiency. Furthermore, the m6A-binding protein heterogeneous nuclear ribonucleoprotein a2b1 (HNRNPA2B1) enhanced the mRNA stability of Shq1. Mechanistically, Virma deficiency resulted in decreased m6A modification on Shq1 mRNA, leading to reduced binding ability of m6A-binding protein HNRNPA2B1 with Shq1, thereby decreasing the mRNA stability of Shq1 and reducing its protein expression level. Downregulation of Shq1 inhibited the PI3K/AKT pathway, thereby suppressing cell proliferation and cell cycle progression, ultimately impeding liver regeneration. In summary, our results demonstrate that VIRMA plays a critical role in promoting liver regeneration by regulating m6A modification, providing valuable insights into the epigenetic regulation during liver regeneration.
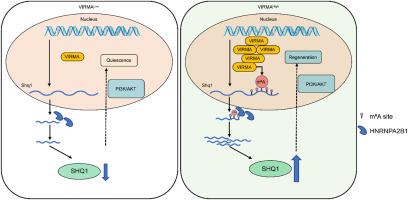
virma介导的SHQ1 m6A修饰通过hnrnpa2b1依赖机制增强肝脏再生
n6 -甲基腺苷修饰(m6A)是一种重要的转录后调控机制,也是真核生物中最丰富、高度保守的RNA表观遗传修饰。先前的研究表明m6A修饰参与多种组织再生过程,包括肝脏再生。病毒样m6A甲基转移酶相关蛋白(VIRMA)是一种具有强大甲基化能力的m6A甲基转移酶。然而,它在肝脏再生中的作用仍然知之甚少。在本研究中,我们使用Cre-loxP系统生成肝脏特异性Virma基因敲除小鼠,并使用阶段性肝切除术(ALPPS)小鼠模型和四氯化碳(CCl4)小鼠模型研究Virma在肝脏再生中的生物学功能。在ALPPS手术后,VIRMA的表达水平迅速上调,在肝脏修复过程中,VIRMA的表达水平逐渐下调。Virma缺乏显著损害肝脏再生能力和破坏细胞周期进程。甲基化RNA免疫沉淀测序(MeRIP-seq)分析显示,Shq1是virma介导的m6A修饰的有效下游靶点。上调Shq1可增强细胞的增殖能力,而AKT特异性抑制剂ipatasertib可减弱细胞的增殖能力。体内补充Shq1可减轻Virma缺乏引起的肝细胞增殖抑制。此外,m6a结合蛋白异质核核糖核蛋白a2b1 (HNRNPA2B1)增强了Shq1 mRNA的稳定性。机制上,Virma缺乏导致m6A对Shq1 mRNA的修饰减少,导致m6A结合蛋白HNRNPA2B1与Shq1结合能力降低,从而降低Shq1 mRNA的稳定性,降低其蛋白表达水平。下调Shq1抑制PI3K/AKT通路,从而抑制细胞增殖和细胞周期进程,最终阻碍肝脏再生。综上所述,我们的研究结果表明,VIRMA通过调节m6A修饰在促进肝脏再生中起着至关重要的作用,为肝脏再生过程中的表观遗传调控提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


