PROTAC-loaded nanocapsules degrading BRD4 for radio-chemotherapy sensitization in glioblastoma
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Glioblastoma (GBM) is a highly aggressive primary brain tumor characterized by poor prognosis. Conventional chemo-radiotherapy demonstrates limited therapeutic efficacy and is often accompanied by significant side effects, largely due to factors such as drug resistance, radiation resistance, the presence of the blood–brain barrier (BBB), and the activation of DNA damage repair mechanisms. There is a pressing need to enhance treatment efficacy, with BRD4 identified as a promising target for increasing GBM sensitivity to therapy. Lacking small molecule inhibitors, BRD4 can be degraded using PROteolysis Targeting Chimera (PROTAC), thereby inhibiting DNA damage repair. To deliver PROTAC, SIAIS171142 (SIS) effectively, we designed a responsive nanocapsule, MPL(SS)P@SIS, featuring GBM-targeting and GSH-responsive drug release. Modified with 1-methyl-l-tryptophan (MLT), nanocapsules facilitate targeted delivery of SIS, downregulating BRD4 and sensitizing GBM cells to radiotherapy and chemotherapy. After intravenous administration, MPL(SS)P@SIS selectively accumulates in tumor tissue, enhancing the effects of radiotherapy and temozolomide (TMZ) by increasing DNA damage and oxidative stress. GSH activates the nanocapsules, triggering BRD4 degradation and hindering DNA repair. In mouse models, the nanosensitizer, combined with TMZ and X-ray irradiation, efficiently inhibited the growth of GBM. These findings demonstrate a novel PROTAC-based sensitization strategy targeting BRD4, offering a promising approach for effective GBM therapy.
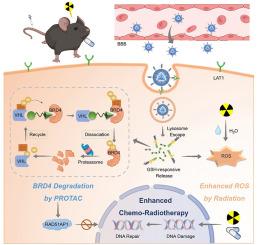
载protac纳米胶囊降解BRD4对胶质母细胞瘤的放化疗增敏
胶质母细胞瘤(GBM)是一种高度侵袭性的原发性脑肿瘤,预后差。传统的放化疗治疗效果有限,往往伴有明显的副作用,主要是由于耐药、耐辐射、血脑屏障(BBB)的存在和DNA损伤修复机制的激活等因素。目前迫切需要提高治疗效果,BRD4被认为是增加GBM对治疗敏感性的有希望的靶点。由于缺乏小分子抑制剂,BRD4可以通过靶向嵌合体(PROteolysis Targeting Chimera, PROTAC)降解,从而抑制DNA损伤修复。为了有效地释放PROTAC, SIAIS171142 (SIS),我们设计了一种响应性纳米胶囊MPL(SS)P@SIS,具有靶向gbm和gsh反应性释放的特点。用1-甲基-l-色氨酸(MLT)修饰的纳米胶囊有助于SIS的靶向递送,下调BRD4并使GBM细胞对放疗和化疗敏感。经静脉给药后,MPL(SS)P@SIS选择性地在肿瘤组织中积累,通过增加DNA损伤和氧化应激,增强放疗和替莫唑胺(TMZ)的效果。谷胱甘肽激活纳米胶囊,引发BRD4降解并阻碍DNA修复。在小鼠模型中,纳米增敏剂联合TMZ和x射线照射可有效抑制GBM的生长。这些发现证明了一种新的基于protac的靶向BRD4的增敏策略,为有效治疗GBM提供了有希望的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


