Small-sized twin-nanoparticles normalize tumor vasculature to enhance tumor accumulation and penetration for potent eradication of cancer stem-like cells
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Cancer stem cells (CSCs) are proposed to account for the progression, metastasis, and recurrence of diverse malignancies. However, the disorganized vasculars in tumors hinder the accumulation and penetration of nanomedicines, posing a challenge in eliminating CSCs located distantly from blood vessels. Herein, a pair of twin-like small-sized nanoparticles, sunitinib (St)-loaded ROS responsive micelles (RM@St) and salinomycin (SAL)-loaded GSH responsive micelles (GM@SAL), are developed to normalize disordered tumor vessels and eradicate CSCs. RM@St releases sunitinib in response to the abundant ROS in the tumor extracellular microenvironment for tumor vessel normalization, which improved intratumor accumulation and homogeneous distribution of small-sized GM@SAL. Sequentially, GM@SAL effectively accesses CSCs and achieves reduction-responsive drug release at high GSH concentrations within CSCs. More importantly, RM@St significantly extends the window of vessel normalization and enhances vessel integrity compared to free sunitinib, thus further amplifying the anti-tumor effect of GM@SAL. The combination therapy of RM@St plus GM@SAL produces considerable depression of tumor growth, drastically reducing CSCs fractions to 5.6% and resulting in 78.4% inhibition of lung metastasis. This study offers novel insights into rational nanomedicines designed for superior therapeutic effects by vascular normalization and anti-CSCs therapy.
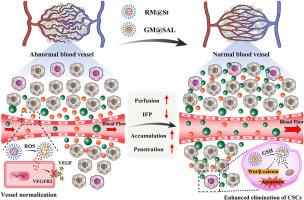
小尺寸的双纳米颗粒使肿瘤血管系统正常化,促进肿瘤的积累和渗透,从而有效地根除癌症干细胞
肿瘤干细胞(CSCs)被认为与多种恶性肿瘤的进展、转移和复发有关。然而,肿瘤中杂乱无章的血管阻碍了纳米药物的积累和渗透,这给消除远离血管的csc带来了挑战。本研究开发了一对双胞胎状的小纳米颗粒,苏尼替尼(St)负载ROS响应胶束(RM@St)和盐霉素(SAL)负载GSH响应胶束(GM@SAL),以使紊乱的肿瘤血管正常化并消除csc。RM@St释放舒尼替尼,以响应肿瘤细胞外微环境中丰富的ROS,促进肿瘤血管正常化,从而改善了小尺寸GM@SAL在肿瘤内的积聚和均匀分布。依次,GM@SAL有效地进入CSCs,并在CSCs内的高GSH浓度下实现还原反应性药物释放。更重要的是,与游离舒尼替尼相比,RM@St显著延长了血管正常化的窗口期,增强了血管的完整性,从而进一步放大了GM@SAL的抗肿瘤作用。RM@St + GM@SAL联合治疗可显著抑制肿瘤生长,使CSCs含量大幅降低至5.6%,肺转移抑制率达到78.4%。该研究为合理的纳米药物设计提供了新的见解,通过血管正常化和抗cscs治疗提供了优越的治疗效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


