Spatiotemporally delivery of Cas9 ribonucleoprotein/DNAzyme logic systems using near-infrared upconversion nanomachine for precise immunotherapy
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Gene therapy, harnessing the power of CRISPR-Cas9 and/or DNAzyme systems, stands as a pivotal approach in cancer therapy, enabling the meticulous manipulation of genes pivotal to tumorigenesis and immunity. However, the pursuit of precise gene therapy encounters formidable hurdles. Herein, a near-infrared upconversion theranostic nanomachine is devised and tailors for CRISPR-Cas9/DNAzyme systems mediate precise gene therapy. An ingenious logic DNAzyme system consists of Chain 1 (C1)/Chain 2 (C2) and endogenous lncRNA is designed. We employ manganese modified upconversion nanoparticles for carrying ultraviolet-responsive C1–PC linker–C2 (C2P) chain and Cas9 ribonucleoprotein (RNP), with outermost coats with hyaluronic acid. Upon reaching tumor microenvironment (TME), the released Mn2+ ions orchestrate a trifecta: facilitating endosomal escape, activating cGAS–STING signaling, and enabling T1-magnetic resonance imaging. Under near-infrared irradiation, Cas9 RNP/C2P complex dissociates, releasing Cas9 RNP into the nucleus to perform gene editing of Ptpn2, while C1/C2 chains self-assemble with endogenous lncRNA to form a functional DNAzyme system, targeting PD-L1 mRNA for gene silencing. This strategy remodels the TME by activating cGAS–STING signaling and dual immune checkpoints blockade, thus realizing tumor elimination. Our theranostic nanomachine armed with the CRISPR-Cas9/DNAzyme logic systems, represents a resourceful and promising strategy for advancing cancer systemic immunotherapy and precise gene therapy.
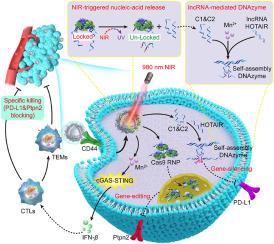
利用近红外上转换纳米机器时空递送Cas9核糖核蛋白/DNAzyme逻辑系统用于精确免疫治疗
基因治疗,利用CRISPR-Cas9和/或DNAzyme系统的力量,是癌症治疗的关键方法,能够对肿瘤发生和免疫的关键基因进行细致的操作。然而,追求精确的基因治疗遇到了巨大的障碍。本文设计了一种近红外上转换治疗纳米机器,为CRISPR-Cas9/DNAzyme系统量身定制,介导精确的基因治疗。设计了一种由链1 (C1)/链2 (C2)和内源性lncRNA组成的巧妙逻辑DNAzyme系统。我们使用锰修饰的上转换纳米颗粒携带紫外线响应的C1-PC连接器- c2 (C2P)链和Cas9核糖核蛋白(RNP),最外层是透明质酸。在到达肿瘤微环境(TME)后,释放的Mn2+离子协调了三个方面:促进内体逃逸,激活cGAS-STING信号,并启用t1磁共振成像。在近红外照射下,Cas9 RNP/C2P复合物解离,释放Cas9 RNP进入细胞核对Ptpn2进行基因编辑,而C1/C2链与内源性lncRNA自组装形成功能性DNAzyme系统,靶向PD-L1 mRNA进行基因沉默。该策略通过激活cGAS-STING信号和双免疫检查点阻断来重塑TME,从而实现肿瘤消除。我们的治疗性纳米机器配备了CRISPR-Cas9/DNAzyme逻辑系统,代表了推进癌症全身免疫治疗和精确基因治疗的一种资源丰富且有前途的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


