Discovery of orally active and serine-targeting covalent inhibitors against hCES2A for ameliorating irinotecan-triggered gut toxicity
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Human carboxylesterase 2A (hCES2A) plays pivotal roles in prodrug activation and hydrolytic metabolism of ester-bearing chemicals. Targeted inhibition of intestinal hCES2A represents a feasible strategy to mitigate irinotecan-triggered gut toxicity (ITGT), but the orally active, selective, and efficacious hCES2A inhibitors are rarely reported. Here, a novel drug-like hCES2A inhibitor was developed via three rounds of structure-based drug design (SBDD) and structural optimization. Initially, donepezil was identified as a moderate hCES2A inhibitor from 2000 US Food and Drug Administration (FDA)-approved drugs. Following two rounds of SBDD and structural optimization, a donepezil derivative (B7) was identified as a strong reversible hCES2A inhibitor. Subsequently, nine B7 carbamates were rationally designed, synthesized and biologically assayed. Among all synthesized carbamates, C3 showed the most potent time-dependent inhibition on hCES2A (IC50 = 0.56 nmol/L), excellent specificity and favorable drug-like properties. C3 could covalently modify the catalytic serine of hCES2A with high selectivity, while this agent also showed favorable safety profiles, high intestinal exposure, and impressive effects for ameliorating ITGT in both human intestinal organoids and tumor-bearing mice. Collectively, this study showcases a rational strategy for developing drug-like and serine-targeting covalent inhibitors against target serine hydrolase(s), while C3 emerges as a promising orally active drug candidate for ameliorating ITGT.
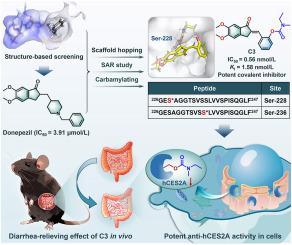
发现针对hCES2A的口服活性和丝氨酸靶向共价抑制剂,以改善伊立替康引发的肠道毒性
人羧酸酯酶2A (hCES2A)在药物前活化和含酯化学物质的水解代谢中起关键作用。靶向抑制肠道hCES2A是缓解伊立替康引发的肠道毒性(ITGT)的一种可行策略,但口服活性、选择性和有效的hCES2A抑制剂很少被报道。本研究通过三轮基于结构的药物设计(SBDD)和结构优化,开发出一种新型药物样hCES2A抑制剂。最初,多奈哌齐被确定为2000种美国食品和药物管理局(FDA)批准的药物中的中度hCES2A抑制剂。经过两轮SBDD和结构优化,多奈哌齐衍生物(B7)被确定为强可逆hCES2A抑制剂。合理设计合成了9种氨基甲酸酯类B7,并进行了生物检测。在所有合成的氨基甲酸酯中,C3对hCES2A表现出最有效的时间依赖性抑制作用(IC50 = 0.56 nmol/L),具有良好的特异性和良好的药物样性质。C3可以高选择性共价修饰hCES2A的催化丝氨酸,同时该药物也显示出良好的安全性,高肠道暴露,并且在改善人类肠道类器官和肿瘤小鼠的ITGT方面具有令人印象深刻的效果。总的来说,本研究展示了一种合理的策略,用于开发针对目标丝氨酸水解酶的药物样和靶向丝氨酸的共价抑制剂,而C3作为一种有希望的口服活性候选药物来改善ITGT。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


