Natural killer cell-derived granzyme B as a therapeutic target for alleviating graft injury during liver transplantation
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Liver transplantation (LT) has become a standard treatment for end-stage liver diseases, and graft injury is intricately associated with poor prognosis. Granzyme B (GZMB) plays a vital role in natural killer (NK) cell biology, but whether NK-derived GZMB affects graft injury remains elusive. Through the analysis of single-cell RNA-sequencing data obtained from human LT grafts and the isolation of lymphocytes from mouse livers following ischemia-reperfusion injury (IRI), we demonstrated that 2NK cells with high expression of GZMB are enriched in patients and mice. Both systemically and liver-targeted depletion of NK cells led to a notable reduction in GZMB+ cell infiltration, subsequently resulting in diminished graft injury. Notably, the reconstitution of Il2rg−/−Rag2−/− mice with purified Gzmb-KO NK cells demonstrated superior outcomes compared to those with wild-type NK cells. Crucially, global knockout of GZMB and pharmacological inhibition exhibited remarkable improvements in liver function in both mouse IRI and rat LT models. Moreover, a phosphorylated derivative of FDA-approved vidarabine was identified as an effective inhibitor of mouse GZMB activity by molecular dynamics, which could provide a potential avenue for therapeutic intervention. Therefore, targeting NK cell-derived GZMB during the LT process suggests potential therapeutic strategies to improve post-transplant outcomes.
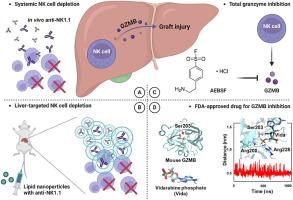
自然杀伤细胞源性颗粒酶B作为减轻肝移植中移植物损伤的治疗靶点
肝移植(LT)已成为终末期肝病的标准治疗方法,移植物损伤与预后不良密切相关。颗粒酶B (Granzyme B, GZMB)在自然杀伤(NK)细胞生物学中起着至关重要的作用,但NK来源的GZMB是否影响移植物损伤尚不清楚。通过分析人LT移植获得的单细胞rna测序数据和小鼠缺血再灌注损伤(IRI)后肝脏淋巴细胞的分离,我们发现患者和小鼠体内都富集了高表达GZMB的2NK细胞。系统性和肝脏靶向NK细胞的消耗导致GZMB+细胞浸润显著减少,随后导致移植物损伤减轻。值得注意的是,与野生型NK细胞相比,纯化的Gzmb-KO NK细胞重建Il2rg−/−Rag2−/−小鼠显示出更好的结果。至关重要的是,GZMB的整体敲除和药物抑制在小鼠IRI和大鼠LT模型中都显示出显著的肝功能改善。此外,经fda批准的阿糖腺苷的磷酸化衍生物通过分子动力学被鉴定为有效的小鼠GZMB活性抑制剂,这可能为治疗干预提供潜在的途径。因此,在移植过程中靶向NK细胞衍生的GZMB提示了改善移植后预后的潜在治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


