Cycle-constrained adversarial denoising convolutional network for PET image denoising: Multi-dimensional validation on large datasets with reader study and real low-dose data
IF 11.8
1区 医学
Q1 COMPUTER SCIENCE, ARTIFICIAL INTELLIGENCE
引用次数: 0
Abstract
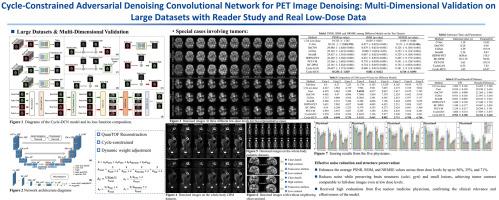
Positron emission tomography (PET) is a critical tool for diagnosing tumors and neurological disorders but poses radiation risks to patients, particularly to sensitive populations. While reducing injected radiation dose mitigates this risk, it often compromises image quality. To reconstruct full-dose-quality images from low-dose scans, we propose a Cycle-constrained Adversarial Denoising Convolutional Network (Cycle-DCN). This model integrates a noise predictor, two discriminators, and a consistency network, and is optimized using a combination of supervised loss, adversarial loss, cycle consistency loss, identity loss, and neighboring Structural Similarity Index (SSIM) loss. Experiments were conducted on a large dataset consisting of raw PET brain data from 1224 patients, acquired using a Siemens Biograph Vision PET/CT scanner. Each patient underwent a 120-seconds brain scan. To simulate low-dose PET conditions, images were reconstructed from shortened scan durations of 30, 12, and 5 s, corresponding to 1/4, 1/10, and 1/24 of the full-dose acquisition, respectively, using a custom-developed GPU-based image reconstruction software. The results show that Cycle-DCN significantly improves average Peak Signal-to-Noise Ratio (PSNR), SSIM, and Normalized Root Mean Square Error (NRMSE) across three dose levels, with improvements of up to 56%, 35%, and 71%, respectively. Additionally, it achieves contrast-to-noise ratio (CNR) and Edge Preservation Index (EPI) values that closely align with full-dose images, effectively preserving image details, tumor shape, and contrast, while resolving issues with blurred edges. The results of reader studies indicated that the images restored by Cycle-DCN consistently received the highest ratings from nuclear medicine physicians, highlighting their strong clinical relevance. A separate set of 50 whole-body PET datasets acquired using the same Biograph Vision scanner, along with an independent set of 245 whole-body pediatric PET datasets acquired using a Siemens Biograph mCT PET/CT scanner at Beijing Friendship Hospital, further validate the generalizability of the proposed model across different imaging centers, scanner types, scanning mode, patient demographics, and anatomical regions.
用于PET图像去噪的周期约束对抗去噪卷积网络:基于阅读器研究和真实低剂量数据的大数据集的多维验证
正电子发射断层扫描(PET)是诊断肿瘤和神经系统疾病的重要工具,但对患者,特别是敏感人群具有辐射风险。虽然减少注射辐射剂量可以减轻这种风险,但它往往会损害图像质量。为了从低剂量扫描中重建全剂量质量的图像,我们提出了一种周期约束的对抗去噪卷积网络(Cycle-DCN)。该模型集成了一个噪声预测器、两个鉴别器和一个一致性网络,并使用监督损失、对抗损失、循环一致性损失、身份损失和邻近结构相似指数(SSIM)损失的组合进行优化。实验采用西门子Biograph Vision PET/CT扫描仪获取的1224例患者的原始PET脑数据进行。每位患者都接受了120秒的脑部扫描。为了模拟低剂量PET条件,使用定制开发的基于gpu的图像重建软件,从缩短的扫描时间30,12,5 s(分别对应于全剂量采集的1/4,1/10和1/24)重建图像。结果表明,在三个剂量水平上,Cycle-DCN显著提高了平均峰值信噪比(PSNR)、SSIM和标准化均方根误差(NRMSE),分别提高了56%、35%和71%。此外,它实现了对比度噪声比(CNR)和边缘保存指数(EPI)值,与全剂量图像紧密一致,有效地保留了图像细节、肿瘤形状和对比度,同时解决了边缘模糊的问题。读者研究结果表明,Cycle-DCN修复的图像始终得到核医学医生的最高评价,突出了其强大的临床相关性。使用同一台Biograph Vision扫描仪获得的50组全身PET数据集,以及北京友谊医院使用西门子Biograph mCT PET/CT扫描仪获得的245组独立的儿童全身PET数据集,进一步验证了所提出模型在不同成像中心、扫描仪类型、扫描模式、患者人口统计学和解剖区域之间的通用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Medical image analysis
工程技术-工程:生物医学
CiteScore
22.10
自引率
6.40%
发文量
309
审稿时长
6.6 months
期刊介绍:
Medical Image Analysis serves as a platform for sharing new research findings in the realm of medical and biological image analysis, with a focus on applications of computer vision, virtual reality, and robotics to biomedical imaging challenges. The journal prioritizes the publication of high-quality, original papers contributing to the fundamental science of processing, analyzing, and utilizing medical and biological images. It welcomes approaches utilizing biomedical image datasets across all spatial scales, from molecular/cellular imaging to tissue/organ imaging.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


