Stereospecific Hydroxyl–Yne Click Polymerization to Selectively Construct High-Performance Plastics and Elastomers
IF 5.2
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
Introducing stereodefined carbon–carbon double bonds into polymeric backbone represents a powerful strategy to regulate the physical and mechanical performance of the resulting materials. Hydroxyl–yne click polymerization has recently emerged as a promising approach for constructing unsaturated polymers, while control over their stereochemistry remains a challenge. Herein, we report a simple and efficient pathway to construct unsaturated poly(vinyl ether ester)s (PVEEs) with absolute control over alkene stereochemistry through hydroxyl–yne click polymerization of trans- or cis-2-butene-1,4-diol as monomer with activated alkynes under mild polymerization conditions. By regulating the alkene stereochemistry, the resultant PVEEs exhibit tunable thermal and mechanical properties, evolving from robust thermoplastics with an ultimate tensile strength of up to 21.6 MPa to tough elastomers with up to 86% elastic recovery. Meanwhile, trans-PVEEs demonstrate superior barrier properties compared to the cis-analogues, which even could be comparable with commercial available polyethylene terephthalate and poly(l-lactide). Importantly, chemical depolymerization of the resultant PVEEs can be accomplished using a binary catalyst system of tetrabutyl titanate and 1,5,7-triazabicyclo[4.4.0]dec-5-ene to selectively recycle the diols and generate functionalized diesters in good to excellent yields.
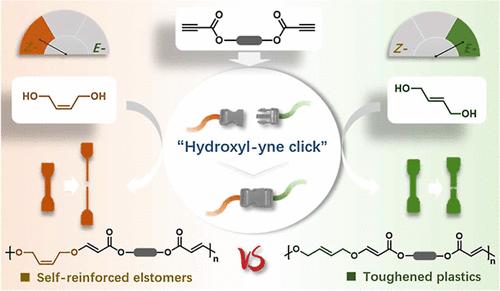
立体定向羟基炔点击聚合选择性构建高性能塑料和弹性体
在聚合物主链中引入立体碳-碳双键是调节材料物理和机械性能的一种有效策略。羟基炔点击聚合近年来成为一种很有前途的不饱和聚合物聚合方法,但对其立体化学的控制仍然是一个挑战。本文报道了在温和的聚合条件下,以反式或顺式-2-丁烯-1,4-二醇为单体,通过羟基炔键聚合,构建了一种简单有效的、完全控制烯烃立体化学的不饱和聚乙烯醚酯(PVEEs)。通过调节烯烃立体化学,合成的pvee具有可调节的热性能和机械性能,从抗拉强度高达21.6 MPa的坚固热塑性塑料演变为弹性回复率高达86%的坚韧弹性体。与此同时,与顺式类似物相比,反式pvee表现出优越的阻隔性能,甚至可以与市售的聚对苯二甲酸乙二醇酯和聚(l-丙交酯)相媲美。重要的是,合成的pvee可以使用钛酸四丁酯和1,5,7-三氮杂环[4.4.0]十二-5-烯二元催化剂体系进行化学解聚,以选择性地回收二元醇并生成具有良好至优异收率的功能化二酯。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


