Neutrophil extracellular traps exacerbate inflammatory arthritis by inhibiting γδ Treg cell differentiation via the AIM2 inflammasome
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Inflammatory arthritis is a set of systemic autoimmune diseases that lead to joint destruction. Recent findings suggest that γδ T cell dysfunction plays a crucial role in the development of inflammatory arthritis, but its underlying mechanisms remain elusive. In this study, we demonstrated that neutrophil extracellular trap (NET) levels were elevated in inflammatory arthritis patients and collagen-induced arthritis (CIA) model mice, which inhibited γδ Treg cell differentiation and contributed to the decreased proportion of γδ Treg cells in these patients and model mice. Inhibition of NET formation with sivelestat (SVT) and CI-amidine restored the proportion of γδ Treg cells and had a therapeutic effect on CIA model mice. In terms of mechanism, the endocytosed NET-associated DNA components bound to an intracellular DNA sensor AIM2, promoting the AIM2 inflammasome activation and the subsequent gasdermin D-mediated mitochondrial dysfunction. This process led to the pathological accumulation of reactive oxygen species, therefore directly inhibiting γδ Treg cell differentiation. Our study reveals the detailed mechanism through which NETs impeded γδ Treg cell differentiation and then exacerbated inflammatory arthritis, suggesting an underlying therapeutic strategy for inflammatory arthritis by targeting the NET-γδ Treg cell axis.
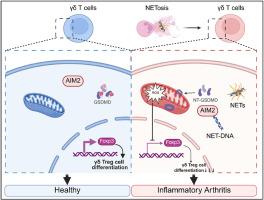
中性粒细胞胞外陷阱通过AIM2炎性小体抑制γδ Treg细胞分化,从而加剧炎性关节炎
炎症性关节炎是一组导致关节破坏的系统性自身免疫性疾病。最近的研究表明,γδ T细胞功能障碍在炎症性关节炎的发展中起着至关重要的作用,但其潜在的机制尚不清楚。在本研究中,我们发现炎症性关节炎患者和胶原诱导关节炎(CIA)模型小鼠的中性粒细胞胞外陷阱(NET)水平升高,抑制了γδ Treg细胞的分化,导致这些患者和模型小鼠的γδ Treg细胞比例下降。西维司他(SVT)和ci -脒抑制NET的形成恢复了γδ Treg细胞的比例,对CIA模型小鼠有治疗作用。就机制而言,内吞的net相关DNA成分结合到细胞内DNA传感器AIM2上,促进AIM2炎性体激活和随后的气皮蛋白d介导的线粒体功能障碍。这一过程导致活性氧的病理积累,从而直接抑制了γδ Treg细胞的分化。我们的研究揭示了NETs阻碍γδ Treg细胞分化进而加重炎性关节炎的详细机制,提示了一种靶向NET-γδ Treg细胞轴治疗炎性关节炎的潜在策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


