Enhancing CO2 adsorption in Ca-based materials via atomic layer deposition of alumina: Mechanisms and stability
IF 4.7
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
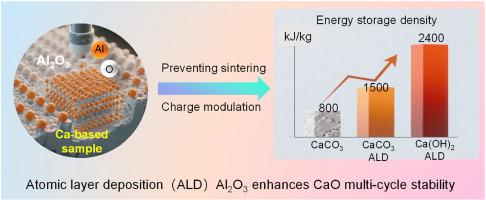
Calcium-based thermochemical energy storage materials (Ca(OH)2/CaCO3) have emerged as promising candidates for industrial waste heat recovery and solar thermal storage applications, owing to their exceptional energy storage density (3.2 GJ/m3) and cost-effectiveness (<50 $/ton). However, their practical implementation faces significant challenges due to performance degradation caused by sintering and particle agglomeration during cyclic operation. This study demonstrates a synergistic approach combining atomic layer deposition (ALD) of Al2O3 coatings and Al3+ doping to enhance CO2 capture in calcium-based materials. Through controlled ALD processing, we achieved nanoscale Al2O3 layers (∼3.6 nm) on CaCO3 substrates. The 20 deposition cycles identified as optimal for balancing Al dispersion and pore accessibility. Materials characterization revealed that Ca(OH)2-derived samples exhibited superior cyclic stability (after 20 cycles) and thermal storage density (∼2400 kJ/kg). Density functional theory calculations uncovered the atomic-scale mechanisms behind these improvements. Al element doping reduced oxygen vacancy formation energy and shortened Al–O bonds by 21 % (1.89 Å vs. Ca–O 2.40 Å), enhancing structural stability. CO2 adsorption on Al-doped surfaces adopted a bent configuration (135°). In humid conditions, H2O co-adsorption further improved performance through a unique [Al–OH2⋯O![]() C
C![]() O⋯Ca] bridge structure, promoting bicarbonate formation.
O⋯Ca] bridge structure, promoting bicarbonate formation.
通过氧化铝原子层沉积增强ca基材料对CO2的吸附:机理和稳定性
基于钙的热化学储能材料(Ca(OH)2/CaCO3)由于其卓越的储能密度(3.2 GJ/m3)和成本效益(50美元/吨),已成为工业废热回收和太阳能蓄热应用的有希望的候选者。然而,由于在循环运行过程中烧结和颗粒团聚导致的性能下降,它们的实际应用面临着重大挑战。该研究展示了一种将Al2O3涂层的原子层沉积(ALD)和Al3+掺杂相结合的协同方法,以增强钙基材料中的CO2捕获。通过控制ALD处理,我们在CaCO3衬底上实现了纳米级Al2O3层(~ 3.6 nm)。这20个沉积循环被认为是平衡铝分散和孔隙可达性的最佳沉积循环。材料表征表明,Ca(OH)2衍生样品具有优异的循环稳定性(经过20个循环)和热存储密度(~ 2400 kJ/kg)。密度泛函理论计算揭示了这些改进背后的原子尺度机制。Al元素掺杂降低了氧空位形成能,缩短了21%的Al - o键(1.89 Å vs. Ca-O 2.40 Å),增强了结构的稳定性。CO2在al掺杂表面上的吸附采用弯曲构型(135°)。在潮湿条件下,H2O共吸附通过独特的[Al-OH2⋯OCO⋯Ca]桥接结构进一步提高了性能,促进了碳酸氢盐的形成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Chemistry and Physics
工程技术-材料科学:综合
CiteScore
8.70
自引率
4.30%
发文量
1515
审稿时长
69 days
期刊介绍:
Materials Chemistry and Physics is devoted to short communications, full-length research papers and feature articles on interrelationships among structure, properties, processing and performance of materials. The Editors welcome manuscripts on thin films, surface and interface science, materials degradation and reliability, metallurgy, semiconductors and optoelectronic materials, fine ceramics, magnetics, superconductors, specialty polymers, nano-materials and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


