Domino C-C/C-N bond-forming strategy for convergent regioselective synthesis of fused pyrimidines using Zn(L-Proline)2: Integrative bioactivity, LFPs imaging and computational modeling
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
A regioselective one-pot hetero-annulation strategy employing a recyclable metallo-organocatalyst was developed for the synthesis of Pyrimido[4,5-d]-2-(methylthio)-[1,2,3]triazolo[1,5-a]pyrimidinedione analogues in good yields. Bio-evaluation via MABA revealed potent antitubercular activity, with the most active members suppressing M. Tuberculosis H37Rv at MIC = 6.25 µg/mL, while anti-inflammatory screening (protein denaturation assay) indicated moderate efficacy with IC50 - 142.88 µg/mL. The synthesized Aggregation-Induced Emission-active compound enabled high-contrast latent fingerprint detection under 365 nm UV, resolving Level I-III ridge minutiae, indicative of its forensic and anticounterfeiting utility. The emissive behaviour was validated by CIE chromaticity mapping and emission spectra. DFT computations elucidated quantum, chemical reactivity parameters, electrostatic potential surfaces and structure-activity corelation, linking the reduced HOMO-LUMO gap with bioactivities. These findings establish Triazolopyrimidinedione frameworks as multifunctional molecular platforms, uniting chemotherapeutics and forensic diagnostics.
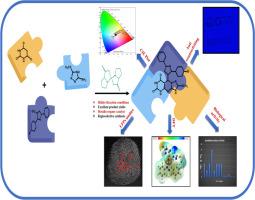
利用Zn(l -脯氨酸)2聚合区域选择性合成融合嘧啶的Domino C-C/C-N成键策略:综合生物活性、LFPs成像和计算模型
采用可回收金属有机催化剂,建立了区域选择性的一锅杂环合成策略,合成了[4,5-d]-2-(甲基硫)-[1,2,3]三唑[1,5- A]嘧啶二酮类似物,收率高。MABA生物评价显示其具有较强的抗结核活性,最活跃的成员抑制结核分枝杆菌H37Rv的MIC值为6.25µg/mL,而抗炎筛选(蛋白质变性试验)显示其作用中等,IC50 - 142.88µg/mL。合成的聚集诱导发射活性化合物能够在365 nm紫外下进行高对比度潜在指纹检测,分辨出I-III级山脊细节,表明其在法医和防伪方面的实用性。通过CIE色度映射和发射光谱验证了其发射行为。DFT计算阐明了量子、化学反应性参数、静电势面和构效关系,将减小的HOMO-LUMO间隙与生物活性联系起来。这些发现确立了三唑嘧啶二酮框架作为多功能分子平台,将化学疗法和法医诊断结合起来。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


