Green synthesis of novel amino acid-coupled 1, 2, 4-triazoles derivatives using lemon juice as a green catalyst: Potential antiproliferative, antimicrobial, DFT computation and molecular docking analysis
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Novel triazole amino acid derivatives (BTPC, CTPC, and NTPC) were synthesized in one-pot reactions and characterized with NMR, UV-Vis, and FT-IR spectroscopy. The synthesized compound chemical structure was optimized using the Density Functional Theory (DFT) B3LYP/6-311++G(d,p) basis set. The Multiwfn program was employed for topological studies such as ELF, LOL, ALIE, RDG, and ALIE (reactive sites of non-covalent interactions). The NBO analysis reveals inter and intra-molecular bond properties. The compounds' antiproliferative activity was tested against MCF-7 and HepG2 human cancer cell lines using the MTT assay. Among the compounds evaluated, NTPC demonstrated the lowest IC50 values for both MCF-7 (4.92 ± 0.78 μM) and HepG2 (6.84 ± 0.81 μM) cell lines, signifying the most cytotoxic effectiveness. The antibacterial properties of the BTPC, CTPC, and NTPC compounds were tested against B. subtilis, S. aureus, E. coli, and P. aeruginosa. NTPC demonstrated the most significant antibacterial activity, with inhibition zones of 23.9 mm against S.aureus and 22.1 mm against B.subtilis. Molecular docking studies confirmed that BTPC has a higher binding affinity for both the breast cancer-associated estrogen receptor (PDB ID: 3ERT) and the liver cancer-associated EGFR kinase (PDB ID: 5UGB) than the other tested compounds CTPC, NTPC, and the reference drugs fluorouracil and sorafenib.
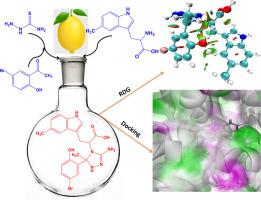
以柠檬汁为绿色催化剂的新型氨基酸偶联1,2,4 -三唑衍生物的绿色合成:潜在的抗增殖、抗菌、DFT计算和分子对接分析
采用一锅法合成了新型三唑类氨基酸衍生物(BTPC、CTPC和NTPC),并用NMR、UV-Vis和FT-IR对其进行了表征。采用密度泛函理论(DFT) B3LYP/6-311++G(d,p)基集对合成化合物的化学结构进行优化。Multiwfn程序用于拓扑研究,如ELF、LOL、ALIE、RDG和ALIE(非共价相互作用的活性位点)。NBO分析揭示了分子间和分子内键的性质。采用MTT法检测化合物对MCF-7和HepG2人癌细胞的抗增殖活性。其中,NTPC对MCF-7(4.92±0.78 μM)和HepG2(6.84±0.81 μM)细胞株的IC50值最低,具有最强的细胞毒作用。研究了BTPC、CTPC和NTPC化合物对枯草芽孢杆菌、金黄色葡萄球菌、大肠杆菌和铜绿假单胞菌的抑菌性能。NTPC对金黄色葡萄球菌和枯草芽孢杆菌的抑制区分别为23.9 mm和22.1 mm。分子对接研究证实,BTPC与乳腺癌相关雌激素受体(PDB ID: 3ERT)和肝癌相关EGFR激酶(PDB ID: 5UGB)的结合亲和力均高于其他被试化合物CTPC、NTPC以及参比药物氟尿嘧啶和索拉非尼。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


