Impact of substituents on bromination sites of (E)-1-[5-methyl-1-aryl-1H-1,2,3-triazol-4-yl]-3-arylprop-2-en-1-ones using N-bromosuccinimide: Synthesis and crystal structure elucidation
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The bromination of (E)-1-[1-aryl-5-methyl-1H-1,2,3-triazol-4-yl]-3-arylprop-2-en-1-ones using N-bromosuccinimide (NBS) in polar protic solvents, such as methanol and ethanol, under acidic conditions was investigated. The bromination site was found to depend on the substituents in chalcones and the reaction conditions. Bromination of phenyl, 4-chlorophenyl, and 4-fluorophenyl-1H-1,2,3-triazolyl chalcones using NBS in methanol occurred at the aliphatic carbon-carbon double bond, producing the corresponding α‑methoxy bromide derivatives with a 90–95 % yield. In contrast, bromination of chalcones with a 4-methoxyphenyl group attached to the unsaturated center under similar conditions resulted in bromination on both the aliphatic double bond and ortho to the methoxy group on the aryl ring, producing the corresponding dibrominated triazoles in 85–90 % yields. When chalcones with a phenyl group on the triazole ring and a phenyl or 4-methoxyphenyl group on the unsaturated center were treated with NBS in ethanol, bromination occurred at the aliphatic double bond, yielding α-ethoxy bromide derivatives in 90–92 % yield. The crystal structures of the synthesized compounds have been determined to confirm their molecular structures, and Hirshfeld surfaces have been utilized to analyze intermolecular interactions within the crystals.
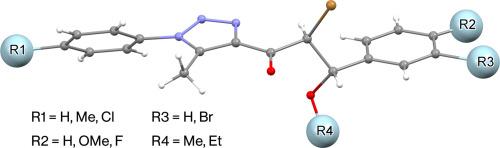
取代基对n-溴代琥珀酰亚胺(E)-1-[5-甲基-1-芳基- 1h -1,2,3-三唑-4-基]-3-芳基-2-烯-1溴化位的影响:合成及晶体结构解析
研究了n-溴琥珀酰亚胺(NBS)在极性质子溶剂(如甲醇和乙醇)中对(E)-1-[1-芳基-5-甲基- 1h -1,2,3-三唑-4-基]-3-芳基-2-烯-1- 1的溴化反应。发现溴化位点取决于查尔酮的取代基和反应条件。苯基、4-氯苯和4-氟苯基- 1h -1,2,3-三唑查尔酮在甲醇中的溴化反应发生在脂肪碳-碳双键处,生成相应的α -甲氧基溴衍生物,收率为90 - 95%。相反,在类似条件下,带4-甲氧基苯基的查尔酮在不饱和中心上的溴化反应会导致脂肪双键和芳基环上的甲氧基的邻位溴化,生成相应的二溴化三唑,产率为85 - 90%。当三唑环上有一个苯基,不饱和中心有一个苯基或4-甲氧基苯基的查尔酮在乙醇中用NBS处理时,脂肪双键发生溴化反应,产率为90 - 92%。合成化合物的晶体结构已被确定,以确认其分子结构,并利用赫希菲尔德表面来分析晶体内的分子间相互作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


