Epigenetic reprogramming via EZH2 inhibition rescues fibroadipose pathogenesis in secondary lymphedema through activating PPARγ signaling
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
Abstract
Background
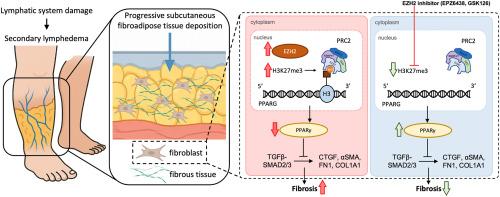
Secondary lymphedema, a progressive disorder characterized by pathological fibroadipose tissue accumulation, is a common problem after cancer treatment and orthopedic surgery. It remains a therapeutic enigma due to its self-perpetuating fibrotic cascade and lack of disease-modifying therapies. While current therapeutic approaches focus on symptom management and volume reduction, they don't address the epigenetic reprogramming driving fibrotic commitment—a process recently linked to enhancer of zeste homolog 2 (EZH2). Although EZH2 inhibitor EPZ6438 has been approved for clinical application, its therapeutic potential in fibroadipose pathogenesis remains unexplored, leaving a critical gap in understanding epigenetic factors in lymphedema progression.
Methods
Human skin tissue was collected from lymphedema patients and normal controls. Histological/immunofluorescence staining and RNA sequencing were performed. In vivo, a mouse hind limb secondary lymphedema model was established by lymphadenectomy. EZH2 inhibitors (EPZ6438, GSK126) were intraperitoneally injected. Skin samples were collected for histological assessment and immuno-staining. In vitro, adipose-derived mesenchymal stem cells (AdMSCs) were treated with transforming growth factor beta 1 (TGFβ1) and EZH2 inhibitors. Western blot, RT-qPCR and ChIP-qPCR were carried out.
Results
Fibrous tissue was observed in lymphedema samples, with concomitant elevation of EZH2 and H3K27me3 levels in the nucleus. RNA sequencing and gene set enrichment analysis (GSEA) revealed significant downregulation of the peroxisome proliferator-activated receptor (PPAR) signaling in lymphedema tissue. Pharmacological inhibition of EZH2 significantly attenuated cutaneous thickening, fibroadipose layer expansion, and collagen deposition in the mouse lymphedema model. PPARγ was induced while phospho-SMAD2/3 activation was suppressed. In TGFβ1 stimulated AdMSCs, EZH2 inhibition upregulated PPARγ expression and inhibited fibrogenic differentiation of the cells.
Conclusion
EZH2 inhibitors exerted potent anti-fibrotic effects in secondary lymphedema though activating PPARγ signaling, offering novel insights and strategies for fibrotic disorders.
The translational potential of this article
This study demonstrated that targeted inhibition of the EZH2-PPARγ axis effectively inhibited fibrogenic differentiation of AdMSCs and reduced fibroadipose tissue in secondary lymphedema, indicating it is a promising strategy for secondary lymphedema treatment, offering novel insights and strategy for musculoskeletal fibrotic disorders.
通过EZH2抑制的表观遗传重编程通过激活PPARγ信号挽救继发性淋巴水肿的纤维脂肪发病机制
背景:继发性淋巴水肿是一种以病理性纤维脂肪组织积累为特征的进行性疾病,是癌症治疗和骨科手术后的常见问题。由于其自我延续的纤维化级联和缺乏改善疾病的治疗方法,它仍然是一个治疗之谜。虽然目前的治疗方法侧重于症状控制和体积减少,但它们并没有解决驱动纤维化的表观遗传重编程——一个最近与zeste同源物2增强子(EZH2)有关的过程。尽管EZH2抑制剂EPZ6438已被批准用于临床应用,但其在纤维脂肪发病机制中的治疗潜力仍未被探索,这在了解淋巴水肿进展中的表观遗传因素方面留下了一个关键的空白。方法采集淋巴水肿患者和正常人的皮肤组织。进行组织/免疫荧光染色和RNA测序。在体内,采用淋巴切除法建立小鼠后肢继发性淋巴水肿模型。EZH2抑制剂(EPZ6438, GSK126)腹腔注射。收集皮肤样本进行组织学评估和免疫染色。在体外,用转化生长因子β1 (tgf - β1)和EZH2抑制剂处理脂肪源性间充质干细胞(AdMSCs)。Western blot、RT-qPCR、ChIP-qPCR检测。结果淋巴水肿标本中可见纤维组织,同时细胞核中EZH2和H3K27me3水平升高。RNA测序和基因集富集分析(GSEA)显示淋巴水肿组织中过氧化物酶体增殖物激活受体(PPAR)信号明显下调。药理抑制EZH2可显著减轻小鼠淋巴水肿模型中皮肤增厚、纤维脂肪层扩张和胶原沉积。诱导PPARγ,抑制phospho-SMAD2/3的激活。在tgf - β1刺激的AdMSCs中,EZH2抑制上调PPARγ表达并抑制细胞的纤维化分化。结论ezh2抑制剂通过激活PPARγ信号通路,在继发性淋巴水肿中发挥了有效的抗纤维化作用,为纤维化疾病的治疗提供了新的见解和策略。本研究表明,靶向抑制EZH2-PPARγ轴可有效抑制继发性淋巴水肿中AdMSCs的纤维化分化,并减少纤维脂肪组织,表明这是一种很有前景的继发性淋巴水肿治疗策略,为肌肉骨骼纤维化疾病提供了新的见解和策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


