Naringin inhibits the osteoblast-osteoclast pyroptosis cascade reaction mediated by accumulated bone marrow adipose tissue in the treatment of postmenopausal osteoporosis
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
Abstract
Background
Excessive expansion of bone marrow adipose tissue (BMAT) is considered to be a crucial factor leading to postmenopausal osteoporosis (PMOP). Conventional osteoporosis drugs mainly focus on inhibiting bone resorption, promoting bone formation or calcium absorption, with less emphasis on regulating BM adiposity. Potential therapeutic agents need to be screened. The objective of this study is to explore the potential mechanisms by which naringin (NG), abundant in citrus fruits, regulates lipid metabolism and exerts bone-protective activities.
Methods
Key pathways and molecular mechanisms underlying NG's amelioration of PMOP symptoms in mice were investigated using RNA sequencing. Molecular docking and surface plasmon resonance were employed to identify the direct targets of NG. The roles of key molecules identified were validated in PMOP through in vivo overexpression or pharmacological inhibition.
Results
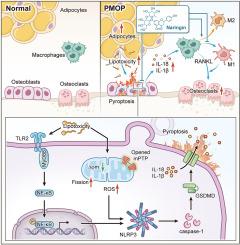
NG treatment improved ovariectomy-induced bone loss and reduced bone marrow fat accumulation. Correspondingly, the co-culture system of adipocytes/osteoblasts suggested impaired osteoblast functionality and alterations in mitochondrial dynamics, which was reversed by NG through its antioxidative effect to restore mitochondrial fission and fusion balance. Furthermore, RNA sequencing results revealed that adipocytes induced osteoblast pyroptosis mediated by NLRP3 inflammasomes. This osteoblast pyroptosis was alleviated following NG to clear ROS or target TLR2 to inhibit MyD88/NF-κB pathway activation. Subsequently, a strong M1 macrophage polarization tendency was observed, as well as accelerated early osteoclast differentiation induced by RANKL, in a co-culture system of pyroptosis-affected osteoblasts and macrophages. However, these changes were reversed by early NG in osteoblasts. Finally, anti-PMOP effect of NG was attenuated in PMOP mice with overexpressed NLRP3, and pharmacological inhibition of NLRP3 alleviated the PMOP symptoms.
Conclusion
NG can regulate osteoblast mitochondrial dynamics disorder through suppression of BMAT-mediated lipotoxicity, and can modulate the MyD88/NF-κB signaling pathway via direct targeting of TLR2, thereby inhibiting the adipocyte-osteoblast-osteoclast pyroptosis cascade.
The translational potential of this article
This study highlights the pivotal role of BMAT-triggered pyroptosis cascades in PMOP pathogenesis and demonstrates NG's therapeutic potential. Our findings position bone marrow adiposity modulation as a promising anti-PMOP strategy, emphasizing the importance of developing natural adiposity regulators like NG for targeted intervention.
柚皮苷抑制骨髓脂肪堆积介导的成骨-破骨细胞焦亡级联反应治疗绝经后骨质疏松症
背景骨髓脂肪组织(BMAT)的过度扩张被认为是导致绝经后骨质疏松症(PMOP)的关键因素。传统的骨质疏松药物主要侧重于抑制骨吸收、促进骨形成或钙吸收,对调节BM肥胖的重视程度较低。需要筛选潜在的治疗药物。本研究的目的是探讨柑橘类水果中富含的柚皮苷(naringin, NG)调节脂质代谢和发挥骨骼保护作用的潜在机制。方法采用RNA测序方法研究NG改善小鼠ppu症状的关键通路和分子机制。利用分子对接和表面等离子体共振技术鉴定了NG的直接靶点。所鉴定的关键分子通过体内过表达或药理抑制在PMOP中得到验证。结果g治疗可改善卵巢切除术所致的骨质流失,减少骨髓脂肪堆积。相应地,脂肪细胞/成骨细胞共培养系统表明成骨细胞功能受损和线粒体动力学改变,NG通过其抗氧化作用恢复线粒体裂变和融合平衡来逆转这一过程。此外,RNA测序结果显示,脂肪细胞诱导的成骨细胞焦亡是由NLRP3炎症小体介导的。NG清除ROS或靶向TLR2抑制MyD88/NF-κB通路激活后,这种成骨细胞凋亡得到缓解。随后,在热致成骨细胞和巨噬细胞共培养系统中,观察到强的M1巨噬细胞极化倾向,以及RANKL诱导的早期破骨细胞分化加速。然而,这些变化被成骨细胞早期NG逆转。最后,NG对NLRP3过表达的ppu小鼠的抗ppu作用减弱,药理抑制NLRP3可减轻ppu症状。结论ng可通过抑制bmat介导的脂肪毒性调节成骨细胞线粒体动力学紊乱,并可通过直接靶向TLR2调控MyD88/NF-κB信号通路,从而抑制脂肪细胞-成骨细胞-破骨细胞焦亡级联反应。本研究强调了bmat触发的焦亡级联反应在ppu发病机制中的关键作用,并证明了NG的治疗潜力。我们的研究结果表明,骨髓脂肪调节是一种很有前途的抗ppu策略,强调了开发天然脂肪调节剂(如NG)进行靶向干预的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


