Discovery of a selective α2C-AR scaffold from a molecular hybridization approach
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
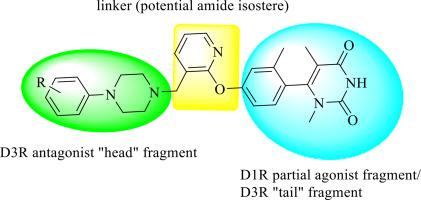
We undertook the rational design and synthesis of a novel series of ligands intended to function as selective dual dopamine D1 receptor (D1R) partial agonists and D3 receptor (D3R) antagonists. The molecular architecture of these compounds was derived by integrating key pharmacophoric features from established D1R partial agonists and D3R antagonists. Specifically, the 6-(2-methylphenyl)-1,5-dimethylpyrimidine-2,4(1H,3H)-dione scaffold was employed as the core “tail” region associated with D1R partial agonism, while various substituted phenyl piperazine moieties were introduced as “head” groups to confer D3R antagonistic activity. A pyridine ring was utilized as a central linker across the series.
Contrary to the intended dopaminergic profile, these compounds exhibited markedly higher binding affinities for α2-adrenergic receptors (α2-ARs) relative to their activity at dopamine receptor subtypes. Several analogues demonstrated potent α2C-AR binding affinities in the low nanomolar range (Ki = 7–30 nM), with moderate selectivity (up to 17-fold) over other α2-AR subtypes. Notably, compounds bearing ortho-substituted aryl groups within the “head” domain generally displayed enhanced α2C-AR binding compared to their para-substituted counterparts.
Molecular docking studies conducted at both α2A-AR and α2C-AR suggested that multiple receptor-ligand interactions contribute to the observed binding profiles. In particular, an anion–pi interaction between Asp131 of α2C-AR and the phenyl ring of the phenyl piperazine “head” moiety was identified as a possible determinant of the increased α2C-AR affinity observed in ortho-substituted analogues.
Given the therapeutic potential of selective α2C-AR targeting in treating various disorders, coupled with the limited availability of clinically approved selective α2C-AR ligands, the discovery of this new scaffold offers new prospects for drug discovery targeting α2C-ARs.
通过分子杂交方法发现选择性α2C-AR支架
我们进行了合理的设计和合成一系列新的配体,旨在作为选择性双多巴胺D1受体(D1R)部分激动剂和D3受体(D3R)拮抗剂。这些化合物的分子结构是通过整合已建立的D1R部分激动剂和D3R拮抗剂的关键药理特征而得到的。具体来说,6-(2-甲基苯基)-1,5-二甲基嘧啶-2,4(1H,3H)-二酮支架被用作与D1R部分拮抗作用相关的核心“尾部”区域,而各种取代的苯基哌嗪部分被引入作为“头部”基团,赋予D3R拮抗活性。一个吡啶环被用作整个系列的中心连接。与预期的多巴胺能谱相反,这些化合物对α2-肾上腺素能受体(α2-ARs)的结合亲和力明显高于它们对多巴胺受体亚型的活性。一些类似物在低纳摩尔范围(Ki = 7-30 nM)表现出强大的α2C-AR结合亲和力,与其他α2-AR亚型相比具有中等选择性(高达17倍)。值得注意的是,在“头”结构域中含有邻取代芳基的化合物通常比其对取代的对应物表现出更强的α2C-AR结合。α2A-AR和α2C-AR的分子对接研究表明,多种受体-配体相互作用有助于观察到的结合谱。特别是,α2C-AR的Asp131与苯基哌嗪“头”部分的苯环之间的阴离子π相互作用被确定为α2C-AR在邻位取代类似物中亲和性增加的可能决定因素。鉴于选择性α2C-AR靶向治疗多种疾病的治疗潜力,加上临床批准的选择性α2C-AR配体有限,这种新的支架的发现为靶向α2C-AR的药物发现提供了新的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


