Metabolic reprogramming represents a targetable mechanism to overcome acquired resistance to venetoclax in acute myeloid leukemia
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-10-01
DOI:10.1016/j.bbadis.2025.168065
引用次数: 0
Abstract
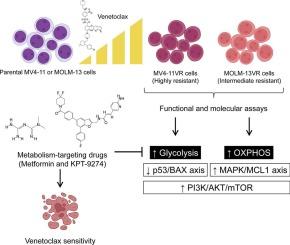
Acute myeloid leukemia (AML) often develops resistance to the BCL2 inhibitor venetoclax through metabolic reprogramming. This study established acquired venetoclax-resistant AML models (MV4-11VR and MOLM-13VR) to explore resistance mechanisms and therapeutic strategies. Cell viability and apoptosis assays revealed robust acquired resistance to venetoclax upon intermittent drug exposure. Metabolic profiling revealed distinct adaptations: MV4-11VR cells favored glycolysis, while MOLM-13VR cells increased oxidative phosphorylation. Proteomic analysis supported these findings, showing pathway enrichment for carbohydrate metabolism in MV4-11VR and aerobic energy production in MOLM-13VR. Despite these differences, both models shared hyperactivation of the PI3K/AKT/mTOR pathway, as shown by RPS6 hyperphosphorylation. Apoptotic regulation also diverged between the cellular models in relation to modulated BCL2-related genes and activation of the MAPK signaling pathway. Targeting these metabolic changes with metformin (a mitochondrial complex I inhibitor) or KPT-9274 (a NAMPT inhibitor) re-sensitized resistant cells to venetoclax. Combination treatments showed strong synergy and near-complete cell elimination. These results highlight metabolic reprogramming as a heterogeneous but targetable resistance mechanism and support combining metabolic inhibitors with BCL2 blockade to treat refractory AML.
代谢重编程是一种克服急性髓性白血病对venetoclax获得性耐药的可靶向机制
急性髓性白血病(AML)通常通过代谢重编程对BCL2抑制剂venetoclax产生耐药性。本研究建立获得性venetoclax耐药AML模型(MV4-11VR和MOLM-13VR),探讨耐药机制和治疗策略。细胞活力和凋亡分析显示,间歇性药物暴露对venetoclax产生了强大的获得性耐药性。代谢分析显示了不同的适应性:MV4-11VR细胞倾向于糖酵解,而MOLM-13VR细胞增加氧化磷酸化。蛋白质组学分析支持了这些发现,显示MV4-11VR中碳水化合物代谢和MOLM-13VR中有氧能量产生的途径富集。尽管存在这些差异,但两种模型都具有PI3K/AKT/mTOR通路的过度激活,如RPS6过度磷酸化所示。与bcl2相关基因的调节和MAPK信号通路的激活有关的细胞模型之间的凋亡调节也存在分歧。二甲双胍(一种线粒体复合物I抑制剂)或KPT-9274(一种NAMPT抑制剂)靶向这些代谢变化,使耐药细胞对venetoclax重新敏感。联合处理表现出较强的协同作用和接近完全的细胞消除。这些结果强调了代谢重编程是一种异质性但可靶向的耐药机制,并支持将代谢抑制剂与BCL2阻断联合治疗难治性AML。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


