A novel 3d-4f two dimensional molecular assembly: synthesis, structure, magnetic, thermal and Hirshfeld surface studies
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
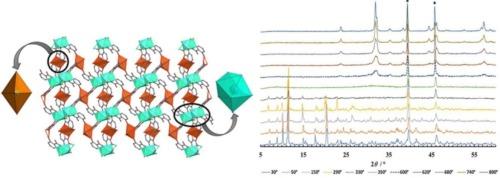
A novel 3d-4f framework has been synthesized with the formula, [Cu2Gd2(L)4(HL)2(H2O)4]n (where, H2L = 3-hydroxypicolinic acid) (1). Compound 1 was characterized by single crystal X-ray diffraction. The structure analysis reveals that the compound possesses 2D network structure. In the structure Gd ions are in square antiprism geometry whereas the copper ions adopt distorted octahedral geometry. The Gd ions are forming a Gd2-dimeric unit (Gd1-Gd1) bridging by the carboxylate oxygen atoms. These units are further bonded by the octahedral copper centers alternately forming a one dimensional zig-zag chain motif. A better overview of the crystal structure of 1 shows that dimeric unit formulated as {Gd2} forms a 1D chain which are linked through the Cu(L) unit and the monoprotonated ligand forming a two dimensional network and this propagates along the crystallographic bc-plane. Compound 1 was subjected for variable temperature magnetic susceptibility measurement. Magnetic study reveals that compound 1 possesses weak ferromagnetic interaction basically due to 3d-4f exchange interaction. In-situ variable temperature PXRD measurement was also carried. PXRD patterns clearly reveal the formation of bimetallic mixed-oxide at higher temperature with particular stoichiometry. Hirshfeld surface (HS) analysis was done to explore the intermolecular contacts present within the molecular crystal.
一种新的3d-4f二维分子组装:合成、结构、磁性、热学和赫希菲尔德表面研究
合成了一种新的3d-4f骨架,分子式为[Cu2Gd2(L)4(HL)2(H2O)4]n (H2L = 3-羟基喹啉酸)(1)。化合物1通过x射线单晶衍射进行了表征。结构分析表明,该化合物具有二维网状结构。在结构中,Gd离子呈方形反棱镜形状,而铜离子呈畸变八面体形状。Gd离子通过羧酸氧原子形成gd2 -二聚体单元(Gd1-Gd1)桥接。这些单元通过八面体铜中心进一步键合,交替形成一维之字形链基序。对1的晶体结构的更好的概述表明,二聚体单位为{Gd2}形成了一个一维链,该链通过Cu(L)单元和单质子化配体连接,形成一个二维网络,并沿着晶体学bc平面传播。化合物1进行了变温磁化率测定。磁性研究表明,化合物1具有弱铁磁相互作用,主要是由于3d-4f交换相互作用。同时进行了现场变温PXRD测量。PXRD图谱清晰地揭示了双金属混合氧化物在高温下的形成,具有特定的化学计量。赫希菲尔德表面(HS)分析是为了探索分子晶体内存在的分子间接触。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


