Redistribution of hydrocarbon and water within graphene mesopores under an external electric field: Evidences from molecular dynamics simulations
IF 2.7
3区 工程技术
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
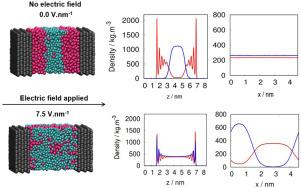
The behavior of confined fluids under external electric fields is central to the development of field-responsive technologies in catalysis and separations. Here, we use Molecular Dynamics simulations to explore the structural response of water–hydrocarbon mixtures (methane, -butane, and -pentane) confined within graphene slit pores, subjected to electric field strengths ranging from 1.5 to 7.5 V. In the absence of an electric field, the system exhibits clear phase separation along the confinement axis, with hydrocarbons preferentially adsorbed near the graphene walls and water localized at the center. As the electric field increases, this organization becomes disrupted, with a lateral redistribution of water molecules and reduced interfacial definition. The spatial rearrangement is confirmed through density profiles along multiple axes, while analysis of the order parameter indicates negligible dipolar alignment of water molecules. Dielectric permittivity calculations reveal anisotropic polarization responses, supporting a mechanism dominated by positional, rather than orientational, field effects. The observed redistribution is significant above 3.0 V and intensifies with stronger fields. These results provide molecular-level insights into the design of nanoconfined systems where electric fields are used to modulate fluid organization. Implications are far-reaching: in electric swing adsorption (ESA), field-induced redistribution offers new levers for controlling selectivity and regeneration; in electrostatic catalysis, particularly Fischer–Tropsch synthesis (FTS), dynamic positioning of polar intermediates such as CO and H2O near catalytic surfaces could fine-tune reaction pathways and improve selectivity. Overall, this work provides a foundational framework for designing electric-field-responsive nanoconfined systems relevant to gas separations and catalytic processes.
外电场下石墨烯介孔内碳氢化合物和水的再分配:来自分子动力学模拟的证据
外加电场作用下受限流体的行为是催化和分离领域场响应技术发展的核心。在这里,我们使用分子动力学模拟来研究限制在石墨烯狭缝孔内的水-烃混合物(甲烷、正丁烷和正戊烷)在1.5到7.5 Vnm−1的电场强度下的结构响应。在没有电场的情况下,该体系沿着约束轴表现出明显的相分离,碳氢化合物优先吸附在石墨烯壁附近,而水则集中在中心。随着电场的增加,这种组织被破坏,水分子的横向重新分配和界面清晰度降低。通过沿多轴的密度分布证实了空间重排,而序参量分析表明水分子的偶极排列可以忽略不计。介质介电常数计算揭示了各向异性极化响应,支持由位置而不是方向场效应主导的机制。在3.0 Vnm−1以上观测到的重分布是显著的,并且随着电场的增强而增强。这些结果为纳米限制系统的设计提供了分子水平的见解,其中电场用于调节流体组织。影响深远:在电振荡吸附(ESA)中,电场诱导的再分配为控制选择性和再生提供了新的杠杆;在静电催化,特别是费托合成(FTS)中,CO和H2O等极性中间体在催化表面附近的动态定位可以微调反应途径并提高选择性。总的来说,这项工作为设计与气体分离和催化过程相关的电场响应纳米限制系统提供了一个基础框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fluid Phase Equilibria
工程技术-工程:化工
CiteScore
5.30
自引率
15.40%
发文量
223
审稿时长
53 days
期刊介绍:
Fluid Phase Equilibria publishes high-quality papers dealing with experimental, theoretical, and applied research related to equilibrium and transport properties of fluids, solids, and interfaces. Subjects of interest include physical/phase and chemical equilibria; equilibrium and nonequilibrium thermophysical properties; fundamental thermodynamic relations; and stability. The systems central to the journal include pure substances and mixtures of organic and inorganic materials, including polymers, biochemicals, and surfactants with sufficient characterization of composition and purity for the results to be reproduced. Alloys are of interest only when thermodynamic studies are included, purely material studies will not be considered. In all cases, authors are expected to provide physical or chemical interpretations of the results.
Experimental research can include measurements under all conditions of temperature, pressure, and composition, including critical and supercritical. Measurements are to be associated with systems and conditions of fundamental or applied interest, and may not be only a collection of routine data, such as physical property or solubility measurements at limited pressures and temperatures close to ambient, or surfactant studies focussed strictly on micellisation or micelle structure. Papers reporting common data must be accompanied by new physical insights and/or contemporary or new theory or techniques.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


