How ionic liquid composition affect Raffinate 1 polymerization in a cationic system?
IF 5.2
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
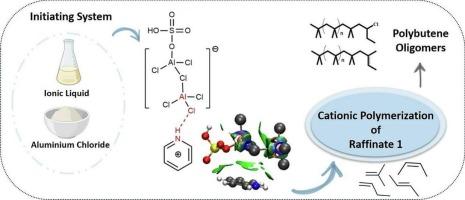
This research investigates the polymerization of Raffinate 1 under mild conditions using AlCl₃-based initiating systems incorporating five different ionic liquids: [MIM]+[HSO₄]−, [Py]+[HSO₄]−, [CycIM]+Cl−, [PVIMCyc]+Cl−, and [PVIM]+[HSO₄]−. The resulting polybutenes were characterized to determine exo-olefin content, number-average molecular weight (Mₙ), and molecular weight dispersity (Ð). The exo-olefin content of the polymers was below 12 %, with Mₙ values ranging from 6000 to 9500 g/mol and Ð values below 2.03. Compared to the neat AlCl₃/ethanol system, the incorporation of ionic liquids generally led to polybutenes with lower Mₙ, comparable or slightly higher Ð, and similar exo-olefin content. Deconvolution of the GPC curves revealed that all polymers featured three distinct active sites. Among these, the site producing low-molecular-weight polymer chains exhibited the lowest activity, contributing less than 10 wt% to the total polymer population. Additionally, Density functional theory calculations via Natural Bond Orbital analysis showed that the average NBO charge on aluminum in the (AlCl₃)₂ dimer complexes was more positive for ILs than for PILs and S-ILs. These NBO charges revealed a nonuniform distribution between the two aluminum centers upon bonding with the hydroxyl group, with an increase in positive charge on the aluminum center coordinating the hydroxyl.
离子液体组成如何影响阳离子体系中的萃余酸盐1聚合?
本研究研究了在温和条件下,以AlCl₃为基础的引发体系,包括五种不同的离子液体:[MIM]+[HSO₄]−,[Py]+[HSO₄]−,[CycIM]+Cl−,[PVIMCyc]+Cl−和[PVIM]+[HSO₄]−,聚合Raffinate 1。对得到的聚丁烯进行了表征,测定了外烯烃含量、数平均分子量(M)和分子量分散度(Ð)。聚合物的外烯烃含量在12%以下,M值在6000 ~ 9500 g/mol之间,Ð值在2.03以下。与纯AlCl₃/乙醇体系相比,离子液体的掺入通常会产生M - n较低的聚丁烯,Ð含量相当或略高,外烯烃含量相似。GPC曲线的反褶积显示,所有聚合物都具有三个不同的活性位点。其中,产生低分子量聚合物链的位点表现出最低的活性,对聚合物总数的贡献不到10%。此外,通过自然键轨道分析的密度泛函理论计算表明,(AlCl₃)2二聚体配合物中铝的平均NBO电荷对ILs比对pil和S-ILs更积极。这些NBO电荷在两个铝中心与羟基成键后呈现不均匀分布,与羟基配位的铝中心正电荷增加。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


