Catalytic asymmetric isomerization/hydroboration of silyl enol ethers
IF 7.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Asymmetric remote hydroboration of olefins has emerged as an efficient strategy for the construction of chiral boronic esters. Conventional asymmetric alkene isomerizations rely on directing groups (OH, NR2, carbonyl) for thermodynamic control via (hyper)conjugation, but their use restricts substrate scope and risks β-heteroatom elimination with transition-metal catalysts. We here reported a catalytic asymmetric isomerization/hydroboration of silyl enol ethers under mild conditions, enabling the efficient synthesis of enantioenriched boryl ethers. The chiral borylether products enable efficient access to valuable 1,n-diols and 1,n-amino alcohols, prevalent in bioactive molecules, and facilitate late-stage functionalization of complex architectures. Preliminary mechanistic studies reveal that this reaction involves a nondissociative chain-walking process and that the β-H elimination may contribute to the rate-limiting step.
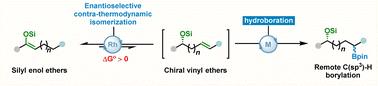
硅烯醇醚的催化不对称异构化/硼化反应
烯烃的不对称远端硼氢化反应已成为一种有效的手性硼酯的构建策略。传统的不对称烯烃异构化依赖于定向基团(OH, NR2,羰基)通过(超)偶联来控制热力学,但它们的使用限制了底物的范围,并且存在被过渡金属催化剂消除β-杂原子的风险。本文报道了在温和条件下催化硅烯醇醚的不对称异构化/硼氢化反应,从而有效合成了富集对映体的硼基醚。手性硼醚产品能够有效地获得生物活性分子中普遍存在的有价值的1,n-二醇和1,n-氨基醇,并促进复杂结构的后期功能化。初步的机制研究表明,该反应涉及非解离链走过程,β-H的消除可能有助于限速步骤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


