Bambusuril Small-Molecule Ionic Isolation Lattices for Exciton Coupled Dimers and Dicationic Fluorophores
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Ionic self-assembly of molecular fluorophores is a promising avenue to program properties into optical materials. Controllable optical energies, lifetimes, exciton hopping and energy transfer have been engineered in small-molecule, ionic isolation lattices (SMILES) by using preferred binding stoichiometries that pair singly charged complexes (−1) with singly charged fluorophores (+1) to isolate them on lattice sites. We hypothesize that these rules can be generalized if charge balance can be expanded from one anion and one cation to pair of each ion, i.e., from 1:1 to 2:2. If true, opportunities emerge to instead bring two cations together as discrete isolated dimers potentially turning on new optical states. We tested these ideas by exchanging cyanostar receptors for bambusuril receptors known to host two chloride anions. When charge matched with two monocationic cyanines in a 2:2 ratio, the crystal structure reveals π-stacked dimers form into isolated emitters that pack charge-by-charge with (bambusuril)·(chloride)2 complexes. New red-shifted emission at 615 nm from cyanine–cyanine dimers and DFT calculations agree with Kasha’s theory of exciton coupling as offset H-dimers. The bambusuril can also host single anions leading to isolation of single cyanines with monomer emission at 507 nm. In addition, we also discovered a SMILES material using dicationic fluorophores (+2) in the form of styryldipyrylium and a new packing stoichiometry with bambusuril. Stoichiometry, anion identity and charge define crystal engineering rules of bambusuril SMILES. Thus, we add valence control to the rules for isolation of discrete dye dimers and inclusion of discrete dicationic dyes in emissive SMILES materials.
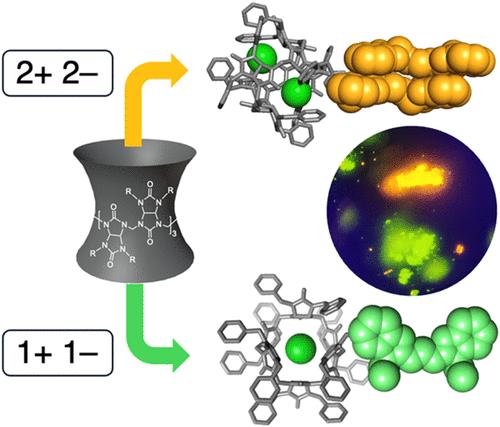
激子偶联二聚体和指示荧光团的Bambusuril小分子离子隔离晶格
分子荧光团的离子自组装是一种很有前途的光学材料编程方法。可控的光能,寿命,激子跳跃和能量转移已经在小分子离子隔离晶格(SMILES)中被设计,通过使用首选结合化学计量,将单电荷配合物(−1)与单电荷荧光团(+1)配对,将它们隔离在晶格位点上。我们假设,如果电荷平衡可以从一个阴离子和一个阳离子扩展到每个离子对,即从1:1扩展到2:2,则这些规则可以推广。如果这是真的,那么就有机会将两个阳离子结合在一起,形成离散的二聚体,可能会开启新的光学状态。我们通过将蓝藻受体与已知含有两个氯阴离子的竹脲受体交换来测试这些想法。当电荷与两个单阳离子菁氨酸以2:2的比例匹配时,晶体结构显示π堆叠二聚体形成孤立的发射体,并与(竹脲)·(氯)2配合物逐电荷封装。花-花二聚体在615 nm处的新红移发射和DFT计算与Kasha的激子耦合理论相一致。竹叶草还可以携带单阴离子,从而在507 nm处以单体发射分离出单花青素。此外,我们还发现了一种使用苯乙烯基二吡啶形式的指示荧光团(+2)的SMILES材料和一种新的竹脲填充化学计量。化学计量学、阴离子恒等式和电荷定义了竹的晶体工程规律。因此,我们将价控制添加到分离染料二聚体的规则中,并在发射smile材料中包含离散指示染料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


