PARK7-driven IGF2BP3–K76 lactylation mediates ferroptosis and HAIC resistance in hepatocellular carcinoma
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Oxaliplatin/5-fluorouracil (OXA/5-FU)-based hepatic artery infusion chemotherapy (HAIC) represents a promising strategy against advanced hepatocellular carcinoma (HCC), yet acquired resistance frequently impedes its efficacy. Here, we identify lactylation of IGF2BP3 at lysine 76 (IGF2BP3–K76lac) as a key driver of HAIC resistance. IGF2BP3–K76lac overexpression enhances chemoresistance in vitro and in vivo. Mechanistically, lactylation at IGF2BP3 K76 strengthens its affinity for m6A-modified FSP1 mRNA, upregulating FSP1 and conferring ferroptosis resistance. Blocking of IGF2BP3–K76lac bolsters OXA/5-FU-induced ferroptosis, disrupts antioxidant defenses, and curbs tumor growth. Moreover, PARK7 functions as a lactyltransferase to facilitate IGF2BP3–K76lac via increasing the binding of lactate at IGF2BP3–K76 site. Finally, blocking antibody targeting IGF2BP3–K76lac was shown to work synergistically with OXA/5-FU to restore chemosensitivity. Taken together, our findings reveal a critical role for the PARK7–IGF2BP3–K76lac–FSP1 axis in HAIC resistance, highlighting IGF2BP3–K76lac as a potential therapeutic target in HCC.
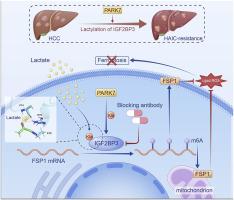
park7驱动的IGF2BP3-K76乳酸化介导肝癌铁凋亡和HAIC耐药。
基于奥沙利铂/5-氟尿嘧啶(OXA/5-FU)的肝动脉输注化疗(HAIC)是一种治疗晚期肝细胞癌(HCC)的有希望的策略,但获得性耐药经常阻碍其疗效。在这里,我们发现IGF2BP3在赖氨酸76处的乳酸化(IGF2BP3- k76lac)是HAIC抗性的关键驱动因素。IGF2BP3-K76lac过表达增强体外和体内化疗耐药。从机制上讲,IGF2BP3 K76的乳酸化增强了其对m6a修饰的FSP1 mRNA的亲和力,上调了FSP1并赋予了铁下沉抗性。阻断IGF2BP3-K76lac增强OXA/5- fu诱导的铁凋亡,破坏抗氧化防御,抑制肿瘤生长。此外,PARK7作为一种乳酸转移酶,通过增加乳酸在IGF2BP3-K76位点的结合来促进IGF2BP3-K76lac。最后,靶向IGF2BP3-K76lac的阻断抗体被证明与OXA/5-FU协同作用以恢复化学敏感性。综上所述,我们的研究结果揭示了PARK7-IGF2BP3-K76lac-FSP1轴在HAIC耐药中的关键作用,强调了IGF2BP3-K76lac是HCC的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


