Betulinaldehyde regulates VSMC phenotypic transition to alleviate vascular hyperplasia through PPARγ/mitochondria/ROS axis
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
Aims
Betulinaldehyde (BA) demonstrates various biological activities both in vivo and in vitro, but its role in vascular remodeling has been little explored. Our network pharmacological analysis suggests that PPARγ serves as a significant regulatory target for BA. This study seeks to clarify the mechanisms associated with BA, thereby offering new perspective targets for addressing vascular remodeling.
Materials and methods
Network pharmacology analysis identified key genes linking BA to vascular remodeling. In vivo effects of BA were evaluated by H&E, immunohistochemical (IHC), and Masson staining. Vascular smooth muscle cells (VSMCs) function was examined using Western blotting, EdU incorporation, wound healing, and transwell assays. Mitochondrial alterations were assessed via electron microscopy, along with JC-1, ROS, and mitoSOX detection.
Key findings
In vivo experiments demonstrated that BA effectively alleviates intimal hyperplasia, collagen deposition, and the increase in the proliferation marker PCNA. Furthermore, BA effectively inhibits VSMC proliferation and migration induced by PDGF-BB. We further found that BA exerts its effects by regulating mitochondrial function through increasing PPARγ expression, which reduces ROS release and ultimately inhibits the progression of vascular remodeling. However, the inhibition or downregulation of PPARγ partially diminishes the therapeutic effects of BA, highlighting its crucial role in this process.
Significance
Our study suggests that BA mitigates vascular remodeling through PPARγ/mitochondria/ROS axis, which uncovers a novel mechanism linking BA to vascular remodeling. And BA's incentive function on PPARγ broadens its application in other diseases.
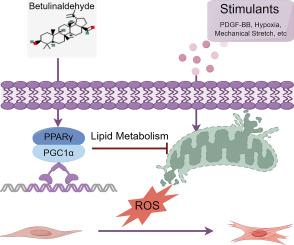
桦木醛通过PPARγ/线粒体/ROS轴调控VSMC表型转变,缓解血管增生。
目的:白桦醛(BA)在体内和体外均表现出多种生物活性,但对其在血管重构中的作用研究甚少。我们的网络药理学分析表明PPARγ是BA的重要调控靶点。本研究旨在阐明与BA相关的机制,从而为解决血管重构提供新的视角靶点。材料和方法:网络药理学分析确定了BA与血管重构相关的关键基因。采用H&E、免疫组化(IHC)和Masson染色评价BA在体内的作用。采用Western blot、EdU掺入、伤口愈合和transwell检测血管平滑肌细胞(VSMC)功能。通过电镜、JC-1、ROS和mitoSOX检测评估线粒体改变。主要发现:体内实验表明,BA能有效缓解内膜增生、胶原沉积和增殖标志物PCNA的升高。此外,BA能有效抑制PDGF-BB诱导的VSMC增殖和迁移。我们进一步发现BA通过增加PPARγ的表达来调节线粒体功能,从而减少ROS的释放,最终抑制血管重构的进展。然而,PPARγ的抑制或下调部分削弱了BA的治疗效果,突出了其在这一过程中的重要作用。意义:我们的研究表明BA通过PPARγ/线粒体/ROS轴减轻血管重构,这揭示了BA与血管重构的新机制。而BA对PPARγ的激励作用拓宽了其在其他疾病中的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


