CEACAM7 enhances oral cancer metastasis by upregulating CD317 expression
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
Aims
Carcinoembryonic antigen-related cell adhesion molecule 7 (CEACAM7) is involved in the regulation of normal cell differentiation. Although numerous studies have demonstrated a strong association between CEACAM7 expression and cancer progression, its specific role in the development and metastasis of oral cancer remains largely uncharacterized.
Materials and methods
Using RNA sequencing technology, transwell migration/invasion assays, western blotting, luciferase reporter, quantitative reverse transcription–polymerase chain reaction (qRT-PCR), and a xenograft model, the effects of CEACAM7 on cell migration in human oral cancer cells were investigated.
Key findings
In this study, analysis of the Gene Expression Omnibus (GEO) database revealed that patients with advanced-stage oral cancer or lymph node metastasis exhibited significantly elevated CEACAM7 expression, which correlated with poorer overall survival. RNA sequencing data further indicated that CEACAM7 overexpression upregulated the expression of CD317. Functional assays showed that CD317 overexpression enhanced the migratory capacity of oral cancer cells, whereas CD317 knockdown suppressed it. Pharmacological inhibition using JNK-IN-8 and PP1 suggested that CEACAM7 may regulate CD317 expression and cell migration via the phosphorylated JNK (p-JNK) and Src (p-Src) signaling pathways. The critical roles of CEACAM7 and CD317 in oral cancer metastasis were further validated using CEACAM7-stable cell lines, in vivo animal experiments, and clinical tissue specimens.
Significance
These findings suggest that CEACAM7 promotes oral cancer metastasis by regulating CD317 through the p-JNK and p-Src pathways. CEACAM7 and CD317 may therefore represent potential therapeutic targets for the treatment of metastatic oral cancer.
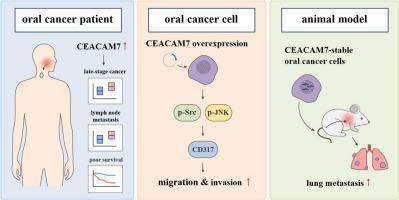
CEACAM7通过上调CD317表达促进口腔癌转移。
目的:癌胚抗原相关细胞粘附分子7 (CEACAM7)参与正常细胞分化的调控。尽管大量研究表明CEACAM7表达与癌症进展之间存在密切关联,但其在口腔癌发生和转移中的具体作用仍未明确。材料与方法:采用RNA测序技术、transwell迁移/侵袭实验、western blotting、荧光素酶报告基因、定量逆转录聚合酶链反应(qRT-PCR)和异种移植模型,研究CEACAM7对人口腔癌细胞迁移的影响。关键发现:在本研究中,基因表达Omnibus (GEO)数据库分析显示,晚期口腔癌或淋巴结转移患者CEACAM7表达显著升高,这与较差的总生存期相关。RNA测序数据进一步表明,CEACAM7过表达上调CD317的表达。功能分析显示,CD317过表达增强了口腔癌细胞的迁移能力,而CD317敲低则抑制了这种能力。使用JNK- in -8和PP1进行药理抑制表明CEACAM7可能通过磷酸化的JNK (p-JNK)和Src (p-Src)信号通路调节CD317的表达和细胞迁移。通过CEACAM7稳定细胞系、体内动物实验和临床组织标本进一步验证了CEACAM7和CD317在口腔癌转移中的关键作用。意义:这些发现提示CEACAM7通过p-JNK和p-Src通路调控CD317促进口腔癌转移。因此,CEACAM7和CD317可能是治疗转移性口腔癌的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


