Fermentative growth decreases the iron demand of Staphylococcus aureus
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Iron (Fe) is an essential nutrient for S. aureus survivability and pathogenesis, but excess Fe can catalyze the formation of toxic oxygen radicals, emphasizing the importance of maintaining proper Fe homeostasis. The essentiality of Fe for bacteria is exploited by host immunity strategies, which employ metal-binding proteins to decrease the availability of metal ions such as Fe. S. aureus responds to Fe limitation using the ferric uptake regulator (Fur) and the Fur protein antagonist (Fpa). During Fe-replete conditions, Fur functions as a transcriptional repressor of target genes. Upon Fe deprivation, Fur repression is relieved with the aid of Fpa, allowing for the increased expression of Fur-regulated genes such as iron uptake systems. We demonstrate that fur inactivation is required during Fe-limited growth and is independent of the described high-affinity Fe uptake systems. Using transcriptomic and metabolomic analyses, we demonstrate that fur inactivation or Fe limitation triggers a decrease in respiration and an increase in fermentation. Triggering fermentative growth allows S. aureus to cope with Fe limitation by having a metabolism less reliant on Fe but allowing for redox balance. Our work provides insight into how S. aureus adapts to iron limitation; a common stress encountered during infection.
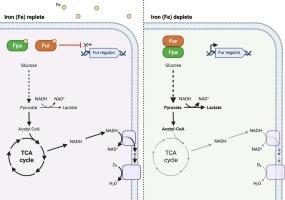
发酵生长降低了金黄色葡萄球菌对铁的需求。
铁(Fe)是金黄色葡萄球菌生存和发病所必需的营养物质,但过量的铁可以催化有毒氧自由基的形成,强调维持适当的铁稳态的重要性。宿主免疫策略利用铁对细菌的重要性,利用金属结合蛋白来减少铁等金属离子的可用性。金黄色葡萄球菌利用铁摄取调节剂(Fur)和铁蛋白拮抗剂(Fpa)对铁限制作出反应。在缺铁条件下,Fur作为靶基因的转录抑制因子发挥作用。在铁缺乏的情况下,在Fpa的帮助下,毛皮抑制得到缓解,允许毛皮调节基因(如铁摄取系统)的表达增加。我们证明,在铁限制生长过程中,皮毛失活是必需的,并且独立于所描述的高亲和力铁摄取系统。利用转录组学和代谢组学分析,我们证明了失活或铁限制会导致呼吸减少和发酵增加。触发发酵生长允许金黄色葡萄球菌通过较少依赖铁的代谢来应对铁限制,但允许氧化还原平衡。我们的工作提供了金黄色葡萄球菌如何适应铁限制的见解;感染过程中常见的压力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


