Neoadjuvant treatment of IBI310 plus sintilimab in locally advanced MSI-H/dMMR colon cancer: A randomized phase 1b study
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Although neoadjuvant immunotherapy showed promising efficacy in locally advanced microsatellite instability-high or mismatch repair-deficient (MSI-H/dMMR) colon cancer, whether dual immune checkpoint inhibition provides additional benefit over anti-PD-1 monotherapy remains unclear. This randomized phase 1b trial (NCT05890742) evaluated a neoadjuvant regimen of IBI310 (anti-cytotoxic T lymphocyte-associated antigen 4 [CTLA-4]) plus sintilimab (n = 52) versus sintilimab monotherapy (n = 49). Surgery was performed in 51 and 45 patients, respectively. The primary endpoint, pathological complete response (pCR) rate, was significantly higher in the combination compared to the monotherapy arm within the modified intent-to-treat (mITT) population (78.4% versus 46.7%, p = 0.0015), with consistent results in the intent-to-treat (ITT) population (76.9% versus 42.9%). Safety in both arms was comparable and manageable without new safety signals. After a median follow-up of 21.4 months, no disease recurrences occurred. One death occurred in each arm due to postoperative complication and adverse events. These findings demonstrate the added benefit of neoadjuvant IBI310 plus sintilimab over sintilimab monotherapy for locally advanced MSI-H/dMMR colon cancer.
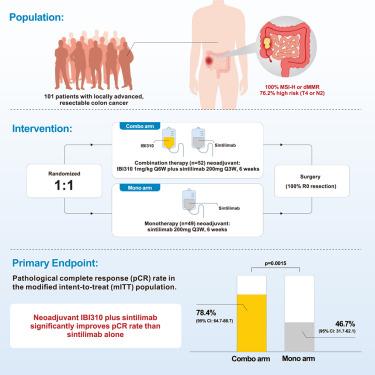
IBI310联合辛替单抗治疗局部晚期MSI-H/dMMR结肠癌:一项随机1b期研究
尽管新辅助免疫治疗在局部晚期微卫星不稳定性高或错配修复缺陷(MSI-H/dMMR)结肠癌中显示出有希望的疗效,但双免疫检查点抑制是否比抗pd -1单药治疗提供额外的益处仍不清楚。这项随机1b期试验(NCT05890742)评估了IBI310(抗细胞毒性T淋巴细胞相关抗原4 [CTLA-4])联合辛替单抗(n = 52)与辛替单抗单药(n = 49)的新辅助方案。分别对51例和45例患者进行了手术。主要终点病理完全缓解(pCR)率在改良意向治疗(mITT)人群中显著高于单药治疗组(78.4%对46.7%,p = 0.0015),意向治疗(ITT)人群的结果一致(76.9%对42.9%)。在没有新的安全信号的情况下,两组的安全性是可比较和可控的。中位随访21.4个月后,无疾病复发。由于术后并发症和不良事件,每组各有1例死亡。这些研究结果表明,新辅助IBI310加辛替单抗治疗局部晚期MSI-H/dMMR结肠癌比辛替单抗治疗有更多的益处。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


