Distinct T cell functions enable efficient immunoediting and prevent tumor emergence of developing sarcomas
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
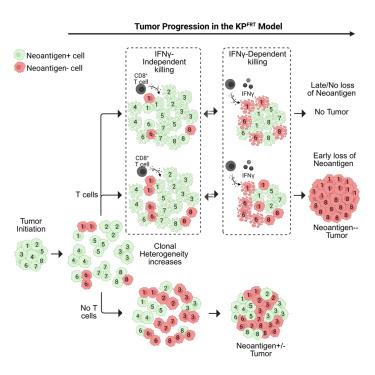
T cells edit tumors by eliminating neoantigen-expressing tumor cells. Yet, how and when this is achieved remains uncertain. Using a murine sarcoma model with fluorescent neoantigens, we found that tumors developed later and in fewer T cell-sufficient mice (∼53% penetrance) than T cell-deficient mice (∼100%). With T cells, all emergent tumor cells had silenced neoantigens, but neoantigen-negative tumor cells were also present in every T cell-deficient mouse. This suggested silencing was necessary but not sufficient for outgrowth. Genetic removal of neoantigens restored tumor penetrance if implemented on day 5 post-tumor initiation, but not day 10, because CD8+ and CD4+ T cells infiltrated the tissue and eliminated most neoantigen-positive and -negative tumor cells within 8 days. Single-cell analyses on day-7 tumors showed oncogenic changes including increased proliferation and T cell-dependent upregulation of the IFNγ-response gene Cd274 (PD-L1). T cell-depletion rescued both neoantigen-positive and -negative cells, while IFNγ blockade rescued only negative cells. This shows that T cells efficiently edit sarcomas of neoantigens and prevent early tumors via IFNγ-independent and IFNγ-dependent (bystander) mechanisms.
不同的T细胞功能能够有效地进行免疫编辑,并防止肿瘤出现发展中的肉瘤
T细胞通过消除表达新抗原的肿瘤细胞来编辑肿瘤。然而,如何以及何时实现这一目标仍不确定。使用带有荧光新抗原的小鼠肉瘤模型,我们发现肿瘤在T细胞充足的小鼠(约53%外显率)中比T细胞缺陷小鼠(约100%)发展得更晚,并且在更少的小鼠中(约53%外显率)。在T细胞中,所有新生肿瘤细胞都有沉默的新抗原,但在每个T细胞缺陷小鼠中也存在新抗原阴性的肿瘤细胞。这表明沉默是必要的,但不足以促进生长。如果在肿瘤发生后第5天进行遗传去除新抗原,则恢复肿瘤外显率,而不是第10天,因为CD8+和CD4+ T细胞浸润组织并在8天内消除了大多数新抗原阳性和阴性的肿瘤细胞。第7天的单细胞分析显示肿瘤发生了致癌变化,包括增殖增加和T细胞依赖的ifn γ反应基因Cd274 (PD-L1)上调。T细胞耗竭可以拯救新抗原阳性和阴性细胞,而IFNγ阻断只能拯救阴性细胞。这表明T细胞通过ifn γ-不依赖和ifn γ-依赖(旁观者)机制有效地编辑新抗原肉瘤并预防早期肿瘤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


