Tom70 prevents fibrotic activation of aortic valve interstitial cells via EPA-activated Autophagic flux
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-09-28
DOI:10.1016/j.bbadis.2025.168063
引用次数: 0
Abstract
Background
Translocase of outer mitochondrial membrane 70 (Tom70) has been implicated in the development of various cardiovascular diseases. However, its role in aortic valve fibrosis, an important feature of calcific aortic valve disease (CAVD), remains unclear.
Objective
This study aims to explore the potential role of Tom70 in aortic valve fibrosis, with a view to providing new insights into the clinical management of CAVD.
Methods
Proteomics, metabolomics and enrichment analysis were used to screen for differential molecules and signaling pathways. Western Blot (WB) and quantitative real-time polymerase chain reaction (qRT-PCR) were employed to detect the expression levels of Tom70 and autophagy-related proteins. Gain- and loss-of-function experiments of Tom70 were conducted both in vivo and vitro to investigate its role in aortic valve fibrosis. Immunofluorescence staining, Masson staining, etc. were used to evaluate fibrosis. Confocal microscopy and transmission electron microscope (TEM) were utilized to detect autophagic flux.
Results
The expression of Tom70 was significantly downregulated in fibrocalcific aortic valve tissues from patients and in aortic valve interstitial cells (AVICs) following TGF-β1 stimulation. In vivo experiments revealed that Tom70 knockout exacerbated aortic valve fibrosis in rat. Mechanistic studies in rat AVICs indicated that impaired autophagic flux accelerates fibrotic activation in AVICs, and Tom70 modulates fibrotic activation through the regulation of autophagic flux. In addition, we identified eicosapentaenoic acid (EPA) as a key regulator of autophagic flux influenced by Tom70.
Conclusion
Overall, our study unveils a novel role of Tom70 in aortic valve fibrosis and elucidates its regulatory relationship with autophagic flux, providing new insights into the molecular mechanisms underlying CAVD.
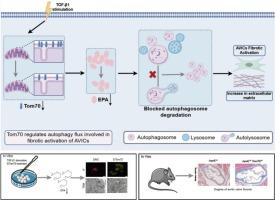
Tom70通过epa激活的自噬通量阻止主动脉瓣间质细胞的纤维化活化。
背景:线粒体外膜70转位酶(Tom70)与多种心血管疾病的发生有关。然而,其在主动脉瓣纤维化(钙化性主动脉瓣疾病(CAVD)的一个重要特征)中的作用尚不清楚。目的:本研究旨在探讨Tom70在主动脉瓣纤维化中的潜在作用,以期为CAVD的临床治疗提供新的见解。方法:采用蛋白质组学、代谢组学和富集分析方法筛选差异分子和信号通路。采用Western Blot (WB)和定量实时聚合酶链反应(qRT-PCR)检测Tom70和自噬相关蛋白的表达水平。在体内和体外进行了Tom70的功能获得和功能丧失实验,以研究其在主动脉瓣纤维化中的作用。采用免疫荧光染色、马松染色等方法评价纤维化程度。利用共聚焦显微镜和透射电子显微镜(TEM)检测自噬通量。结果:TGF-β1刺激后,患者纤维钙化主动脉瓣组织和主动脉瓣间质细胞(AVICs)中Tom70的表达明显下调。体内实验显示Tom70基因敲除加重了大鼠主动脉瓣纤维化。大鼠AVICs的机制研究表明,自噬通量受损加速AVICs的纤维化激活,Tom70通过调节自噬通量调节纤维化激活。此外,我们发现二十碳五烯酸(eicosapentaenoic acid, EPA)是受Tom70影响的自噬通量的关键调节因子。结论:总的来说,我们的研究揭示了Tom70在主动脉瓣纤维化中的新作用,并阐明了其与自噬通量的调节关系,为CAVD的分子机制提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


