Cgas deficiency promotes tumor growth by supporting B cell persistence and angiogenesis
IF 2.9
4区 医学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
The cGAS sensor activates the STING/IFN signaling pathway, which is crucial for antiviral and antitumor responses. This study aims to investigate the cGAS-mediated immune responses in tumorigenesis using the MC-38 tumor model. MC38-tumor models were established in wild-type (WT) and Cgas-deficient mice to investigate immunophenotypes and cellular mechanisms involved in tumor progression. Cgas−/− mice exhibited significantly larger tumors and reduced survival compared to WT mice. Tumors in Cgas−/− mice showed increased fibrosis and neovascularity. WT mice mounted a more robust T-cell-mediated antitumor response, with higher levels of NK and effector T cells, while Cgas−/− mice showed an expansion of B cells, including regulatory B cells producing IL-10. B cells from tumor-bearing Cgas−/− mice demonstrated enhanced survival in the tumor-conditioned medium than those from WT mice. B cell depletion significantly reduced tumor size in WT mice but had minimal effect in Cgas−/− mice, where fibrosis and tumor vasculature persisted. Notably, despite B cell depletion, B cells remained in the tumors of Cgas−/− mice, in contrast to WT mice, where depletion correlated with increased CD8+ T cell infiltration. Upregulation of Tgfb1, Tlr7, Tlr9, and Tnfrsf13c in tumors of Cgas−/− mice suggested a tumor microenvironment (TME) that promotes B cell survival. Furthermore, Cgas−/− B cells promoted angiogenesis, as indicated by enhanced endothelial tube formation. cGAS deficiency fosters tumor growth by reducing the antitumor response, promoting a pro-tumor microenvironment, and supporting B cell survival. The Cgas−/− B cells enhance angiogenesis and are resistant to B cell depletion, contributing to tumor progression.
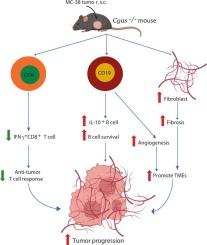
Cgas缺乏通过支持B细胞持久性和血管生成来促进肿瘤生长。
cGAS传感器激活STING/IFN信号通路,这对抗病毒和抗肿瘤应答至关重要。本研究旨在利用MC-38肿瘤模型研究cgas介导的肿瘤发生免疫反应。在野生型(WT)和cgas缺陷小鼠中建立mc38肿瘤模型,研究参与肿瘤进展的免疫表型和细胞机制。与WT小鼠相比,Cgas-/-小鼠表现出明显更大的肿瘤和更低的存活率。Cgas-/-小鼠的肿瘤显示纤维化和新生血管增加。WT小鼠表现出更强的T细胞介导的抗肿瘤反应,具有更高水平的NK细胞和效应T细胞,而Cgas-/-小鼠表现出B细胞的扩增,包括产生IL-10的调节性B细胞。来自携带肿瘤的Cgas-/-小鼠的B细胞在肿瘤条件培养基中的存活率高于来自WT小鼠的B细胞。B细胞耗竭在WT小鼠中显著减小肿瘤大小,但在Cgas-/-小鼠中作用最小,其中纤维化和肿瘤血管系统持续存在。值得注意的是,尽管B细胞耗尽,但Cgas-/-小鼠的肿瘤中仍存在B细胞,与WT小鼠相反,后者的消耗与CD8+ T细胞浸润增加相关。Cgas-/-小鼠肿瘤中Tgfb1、Tlr7、Tlr9和Tnfrsf13c的上调提示肿瘤微环境(tumor microenvironment, TME)促进B细胞存活。此外,Cgas-/- B细胞促进血管生成,如内皮管形成增强所示。cGAS缺乏通过降低抗肿瘤反应、促进促肿瘤微环境和支持B细胞存活来促进肿瘤生长。Cgas-/- B细胞增强血管生成,抵抗B细胞耗竭,促进肿瘤进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular immunology
生物-免疫学
CiteScore
8.20
自引率
2.30%
发文量
102
审稿时长
30 days
期刊介绍:
Cellular Immunology publishes original investigations concerned with the immunological activities of cells in experimental or clinical situations. The scope of the journal encompasses the broad area of in vitro and in vivo studies of cellular immune responses. Purely clinical descriptive studies are not considered.
Research Areas include:
• Antigen receptor sites
• Autoimmunity
• Delayed-type hypersensitivity or cellular immunity
• Immunologic deficiency states and their reconstitution
• Immunologic surveillance and tumor immunity
• Immunomodulation
• Immunotherapy
• Lymphokines and cytokines
• Nonantibody immunity
• Parasite immunology
• Resistance to intracellular microbial and viral infection
• Thymus and lymphocyte immunobiology
• Transplantation immunology
• Tumor immunity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


