Tetramethylguanidine-Functionalized Novel and Recyclable Nanocatalyst for the Synthesis of Pyrazolophthalazinediones
IF 2.6
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
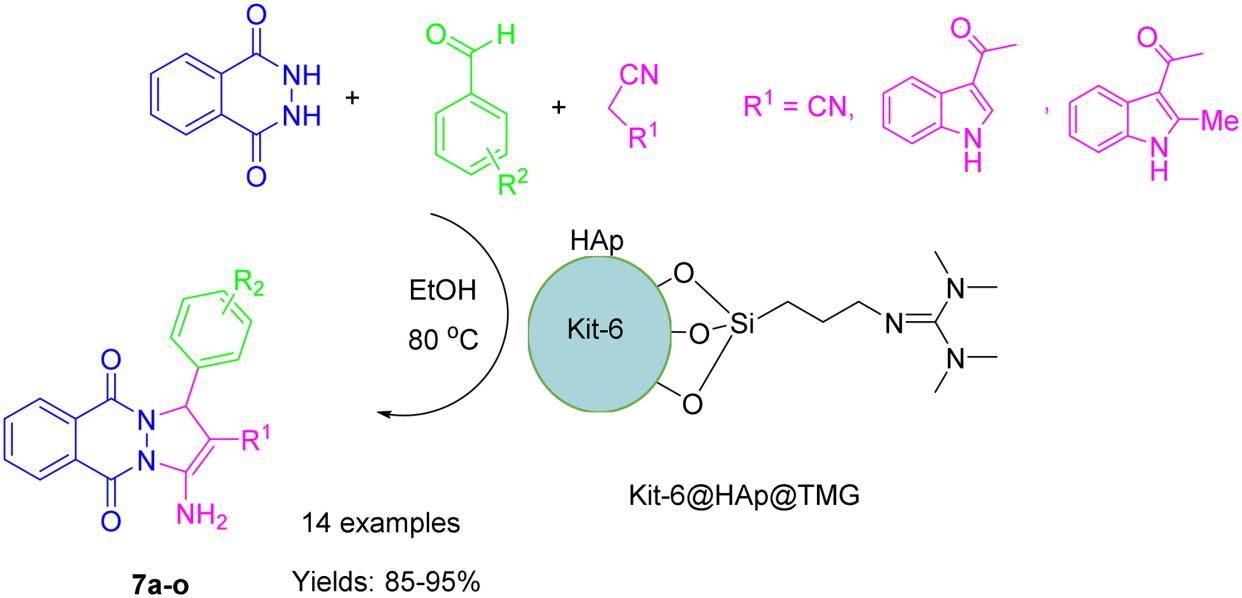
The synthesis of pyrazolophthalazinediones has so far been carried out using acid, base, and ionic liquids catalysts, which in some cases are not easily recyclable, but in this work, three-component synthesis was carried out using a recyclable nanocatalyst. Therefore, a novel protocol was devised for the synthesis of pyrazolophthalazinediones by the reaction of phthalhydrazide, aryl aldehyde and 3-(1H-indol-3-yl)-3-oxopropanenitrile derivatives or malononitrile in ethanol and in the presence of newly synthesized Kit-6@HAp@Si(CH2)3-1,1,3,3-tetramethylguanidine (Kit-6@HAp@TMG) nanocatalyst with high to excellent yields (84–95%). The structure of the catalyst was determined using various analytical techniques, including FT-IR, XRD, SEM, EDX, Mapping, TEM, and TGA. The structures of the products were confirmed by spectroscopic analyses (13C NMR,1H NMR, and FT-IR) and elemental analyses. The present method offers advantages, such as simplicity, high yields, and shorter reaction time, Easy method for separating the catalyst from the synthesized product, avoiding high temperatures and using low-risk solvents, atom economy, and catalyst recyclability up to four times without significant decrease in catalytic activity, which aligns with green chemistry principles.
四甲基胍功能化的新型可回收纳米催化剂合成吡唑苯二酮
迄今为止,吡唑苯并二酮的合成主要是使用酸、碱和离子液体催化剂,这些催化剂在某些情况下不容易回收,但在本研究中,使用可回收的纳米催化剂进行了三组分合成。因此,设计了一种新的方案,在新合成的Kit-6@HAp@Si(CH2)3-1,1,3,3-四甲基胍(Kit-6@HAp@TMG)纳米催化剂的存在下,通过邻苯二肼、芳基醛和3-(1h -吲哚-3-基)-3-氧丙腈衍生物或丙二腈在乙醇中反应合成吡唑酞吡啶二酮,收率高至优异(84-95%)。采用FT-IR、XRD、SEM、EDX、Mapping、TEM和TGA等多种分析技术对催化剂的结构进行了表征。产物的结构经13C NMR、1H NMR、FT-IR等光谱分析和元素分析证实。该方法具有简单、产率高、反应时间短、催化剂与合成产物分离简便、避免高温和使用低风险溶剂、原子经济、催化剂可循环使用4次而催化活性不显著降低等优点,符合绿色化学原理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polycyclic Aromatic Compounds
化学-有机化学
CiteScore
3.70
自引率
20.80%
发文量
412
审稿时长
3 months
期刊介绍:
The purpose of Polycyclic Aromatic Compounds is to provide an international and interdisciplinary forum for all aspects of research related to polycyclic aromatic compounds (PAC). Topics range from fundamental research in chemistry (including synthetic and theoretical chemistry) and physics (including astrophysics), as well as thermodynamics, spectroscopy, analytical methods, and biology to applied studies in environmental science, biochemistry, toxicology, and industry. Polycyclic Aromatic Compounds has an outstanding Editorial Board and offers a rapid and efficient peer review process, as well as a flexible open access policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


