Chemical characterization and toxicological evaluation of acrylic-based dental implant devices used for different purposes within the scope of ISO 10993
IF 3.5
4区 医学
Q1 MEDICINE, LEGAL
引用次数: 0
Abstract
Medical devices have the potential to release chemical constituents, either as residual manufacturing additives or as leachable under clinical conditions, which may pose potential toxicological risks. Therefore, comprehensive chemical characterization is crucial to identify and quantify extractable compounds that could migrate from the device during clinical use, in accordance with ISO 10993 standards.
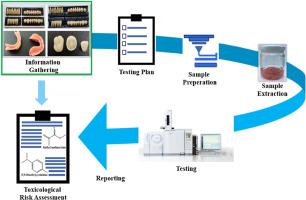
This study provides a comprehensive chemical characterization and toxicological risk assessment of acrylic-based dental devices intended for various clinical applications. By adhering to ISO 10993–12:2021 for extraction protocols and employing both polar (water) and apolar (50:50 ethanol-water) solvents, leachable were analyzed after 72-h incubations at 50 °C. Fourier Transform Infrared Spectroscopy (FTIR) confirmed the use of polymethyl methacrylate (PMMA) in device matrices, while thermogravimetric analysis (TGA) revealed the presence of inorganic pigments.
Extractable compounds were identified by using a broad range of analytical techniques-including Gas Chromatography-Mass Spectrometry (GC-MS), High Performance Liquid Chromatography (HPLC), UV–VIS–NIR Spectrophotometry because of extractable studies, Liquid Chromatography – Tandem Mass Spectrometry (LC-MS/MS) and Inductively Coupled Plasma–Optical Emission Spectrometry (ICP-OES) techniques, detecting compounds such as phenyl benzoate, N,N-dimethyl-p-toluidine, benzoyl peroxide, ethylene glycol dimethacrylate, and benzoic acid. Toxicological risk assessments were subsequently conducted in accordance with ISO 10993–17:2023, demonstrating that all tested devices were biocompatible and posed no toxicological hazard under intended clinical conditions.
ISO 10993范围内用于不同用途的丙烯酸基牙科植入装置的化学特性和毒理学评价。
医疗器械有可能释放化学成分,作为残留的制造添加剂或在临床条件下可浸出,这可能构成潜在的毒理学风险。因此,根据ISO 10993标准,全面的化学表征对于识别和量化临床使用期间可能从设备迁移的可提取化合物至关重要。本研究提供了用于各种临床应用的丙烯酸基牙科器械的综合化学特性和毒理学风险评估。通过遵循ISO 10993- 12:21 21提取方案,并采用极性(水)和极性(50:50乙醇-水)溶剂,在50°C下培养72小时后分析可浸出性。傅里叶变换红外光谱(FTIR)证实了在器件基质中使用了聚甲基丙烯酸甲酯(PMMA),而热重分析(TGA)显示了无机颜料的存在。可提取化合物通过广泛的分析技术进行鉴定,包括气相色谱-质谱(GC-MS),高效液相色谱(HPLC),紫外-可见-近红外分光光度法(因为可提取研究),液相色谱-质谱(LC-MS/MS)和电感耦合等离子体质谱(ICP-OES)技术,检测化合物如苯甲酸苯酯,N,N-二甲基-对甲苯胺,过氧化苯甲酰,乙二醇二甲基丙烯酸酯,还有苯甲酸。随后根据ISO 10993-17:2023进行毒理学风险评估,证明所有测试设备具有生物相容性,并且在预期的临床条件下不构成毒理学危害。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.70
自引率
8.80%
发文量
147
审稿时长
58 days
期刊介绍:
Regulatory Toxicology and Pharmacology publishes peer reviewed articles that involve the generation, evaluation, and interpretation of experimental animal and human data that are of direct importance and relevance for regulatory authorities with respect to toxicological and pharmacological regulations in society. All peer-reviewed articles that are published should be devoted to improve the protection of human health and environment. Reviews and discussions are welcomed that address legal and/or regulatory decisions with respect to risk assessment and management of toxicological and pharmacological compounds on a scientific basis. It addresses an international readership of scientists, risk assessors and managers, and other professionals active in the field of human and environmental health.
Types of peer-reviewed articles published:
-Original research articles of relevance for regulatory aspects covering aspects including, but not limited to:
1.Factors influencing human sensitivity
2.Exposure science related to risk assessment
3.Alternative toxicological test methods
4.Frameworks for evaluation and integration of data in regulatory evaluations
5.Harmonization across regulatory agencies
6.Read-across methods and evaluations
-Contemporary Reviews on policy related Research issues
-Letters to the Editor
-Guest Editorials (by Invitation)

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


